The advance, reported in Nature Chemistry, has been made by BISfuel, a US Department of Energy funded Energy Frontier Research Center in the Department of Chemistry and Biochemistry at Arizona State University(ASU).
‘Initially, our artificial leaf did not work very well, and our diagnostic studies on why indicated that a step where a fast chemical reaction had to interact with a slow chemical reaction was not efficient,’ said ASU chemistry professor Thomas Moore in a statement. ‘The fast one is the step where light energy is converted to chemical energy, and the slow one is the step where the chemical energy is used to convert water into its elements viz. hydrogen and oxygen.’
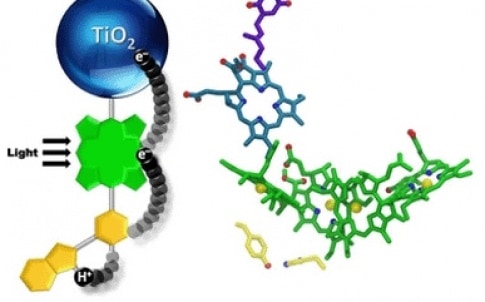
The researchers took a closer look at how nature had overcome a related problem in the part of the photosynthetic process where water is oxidised to yield oxygen.
‘We looked in detail and found that nature had used an intermediate step,’ said Moore. ‘This intermediate step involved a relay for electrons in which one half of the relay interacted with the fast step in an optimal way to satisfy it, and the other half of the relay then had time to do the slow step of water oxidation in an efficient way.’
They then designed an artificial relay based on the natural one and were rewarded with a major improvement.
Seeking to understand what they had achieved, the team used X-ray crystallography and optical and magnetic resonance spectroscopy techniques to determine the local electromagnetic environment of the electrons and protons participating in the relay, and with the help of theory - proton coupled electron transfer mechanism - identified a unique structural feature of the relay. This was an unusually short bond between a hydrogen atom and a nitrogen atom that facilitates the correct working of the relay.
They also found subtle magnetic features of the electronic structure of the artificial relay that mirrored those found in the natural system.
ASU chemistry professors involved in this specific project include Thomas Moore, Devens Gust, Ana Moore and Vladimiro Mujica. The department is a unit of the College of Liberal Arts and Sciences. Key collaborators in this work are Oleg Poluektov and Tijana Rajh from Argonne National Laboratory.





Nanogenerator consumes CO2 to generate electricity
Whoopee, they've solved how to keep a light on but not a lot else.