The researchers, part of an ‘artificial pancreas consortium’ at the university, have made progress on integrating an insulin pump mechanism with a continuous blood-glucose monitoring system, a feat which has so far seemed to be an insurmountable problem.
An artificial pancreas would make life significantly easier for people suffering from type-1 diabetes, the variant of the disease where the body produces no insulin of its own.
Currently, sufferers have to test the level of glucose in their blood several times per day by pricking a finger and feeding a drop of blood into a hand-held meter; they must then calculate how much insulin they need to inject based on what they have been eating, how active they expect to be and several other factors. This means that their insulin requirements can vary widely: one day they might get through three times their average dosage, the next day only a third of it. Getting the dosage wrong risks hypo- or hyperglycaemia (too little or too much sugar), both of which cause cumulative damage to nerves and blood vessels.
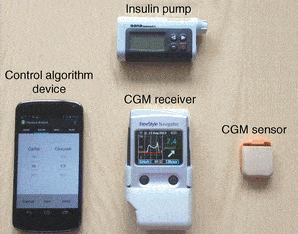
An artificial pancreas would eliminate this problem, constantly monitoring glucose levels and administering insulin to compensate, in the same way as a normal functioning pancreas does in a non-diabetic person. But it’s not a simple matter. Even the fast-acting insulin analogues used by type-1 diabetics do not reach their peak level in the bloodstream until 30min to 2 hours after injection, for example. This and other problems make the software needed to control so-called closed-loop systems, where monitoring and dosage are integrated, difficult to programme to maintain accurate glucose control.
In a paper in the journal Diabetologica, Hood Thabit and Roman Havorka of the consortium explain how they have been testing their closed-loop system in 6 to 24 month-long clinical trials, in a variety of settings including patients in their own homes and children at residential ‘diabetes camps’ where they are trained in how to manage their condition.
Compared with conventional test-and-inject management systems, they found that patient using the pump spent less time with abnormal glucose levels; in the longest trial, over three months, glucose levels were maintained within the healthy range 11 per cent longer per day.
The artificial pancreas is about the size of a mobile phone and worn outside the body, with a blood glucose sensor communicating with the device wirelessly – which in itself entails security protocols ensuring that the system cannot be hacked to access data or interfere with transmissions.
One of the important strands of the team’s current research is to determine which groups of people might benefit the most from the technology; for example, pregnant women, very young children, or people in hospital suffering from hypoglycaemia or its after-effects. The researchers conclude that "Given the challenges of beta-cell transplantation [where healthy pancreas cells are implanted, entailing major surgery, immune-suppressant medication and other risks], closed-loop technologies are, with continuing innovation potential, destined to provide a viable alternative for existing insulin pump therapy and multiple daily insulin injections.”
The US FDA is currently reviewing one system, with approval possible by the end of next year; in the UK, the National Institute of Health Research has reported that automated closed-loop systems may be expected to appear in the (European) market by the end of 2018.





Nanogenerator consumes CO2 to generate electricity
Whoopee, they've solved how to keep a light on but not a lot else.