The need to reduce carbon emissions in a bid to negate the impacts of climate change are only too apparent but there has yet to be a single country that has been able to neglect the carbon-emitting fossil fuels the world has become so dependent on.
Renewable energy sources are out there in abundance but the truth of the matter is they aren’t yet efficient enough to compete with relatively low priced fossil fuels. According to a report published by the Royal Academy of Engineering, the price of producing electricity from a gas power station is 2.2p per kWh, while the price of electricity from an offshore wind farm is 7.2p per kWh.
Until the price of renewable energy is reduced, or their efficiency is increased, there will remain a need to harness the carbon emitting power plants, of which there were 351 in operation in the UK at the end of May 2011. In the short term, carbon capture and storage (CCS) technologies could hold the answer.
CCS involves capturing carbon dioxide at source, compressing it, and then storing it underground in porous rocks, usually in the ocean. There are numerous techniques and technologies that can be used to do this and the vast majority of the individual elements in the CCS process are used today in the oil, gas and chemical sectors.
The International Energy Agency (IEA) has called for 1,500 full-scale CCS plants to be in operation by 2035. Just a slight increase on the eight we have in operation worldwide today.
But how do we capture CO2 to begin with? One way is to absorb it with the help of a sorbent material.
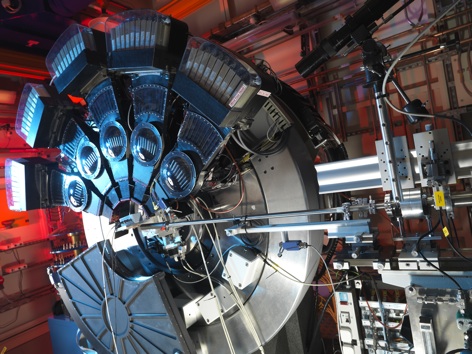
Scientists at Leeds University are using the facilities at Diamond Light Source — the UK’s national synchotron - to measure how much CO2 certain materials can absorb. They specifically used the ultra high-resolution powder diffraction beamline, I11, to study the carbon capture of calcium oxide (CaO) based materials. These materials have a large range of applications including pre- and post-combustion carbon capture technologies. In addition, they are also cheap, abundant, and boast fast reaction rates during the chemical processes that are used during capture.

‘CaO is a natural CO2 storage material in the form of limestone and dolomite,’ said joint project leader Valerie Dupont from the School of Process, Environmental and Materials Engineering at Leeds University.
According to the Leeds University researchers, the CaO based materials capture CO2 between 400 — 800°C by forming calcium carbonate (CaCO3). When this is regenerated it releases CO2 that can easily be compressed and stored.
‘We were tracking CO2 capture of CaO (calcium oxide/lime) and Ca(OH)2 (portlandite) using x-ray diffraction,’ said the other project leader, Prof Tim Comyn from Leeds University’s Faculty of Engineering. ‘In essence, lime and portlandite capture CO2 to form calcium carbonate (CaCO3), or calcite.’
The team used the synchotrons’s intense X-rays to study the carbon capture and hydration process in CaO based materials on the nanoscale. No other facility in the UK is able to provide the resolution that the facilities at Diamond Light Source can achieve.
After studying the process, the Leeds team found that pure calcium carbonate is converted into two parts CaCO3 and one part CaO when it is reacted with CO2.
They also realised that there are certain calcium oxide compounds, which are even more effective when it comes to capturing carbon dioxide.
‘Pure portlandite (Ca(OH)2) was able to transform completely to CaCO3, therefore making it 100 per cent efficient,’ said Comyn.
The latter are cheap and abundant all over the world, but for CaO to claim any net CO2 capture, it needs to lose its initial CO2 content. This is done by exposing it to temperatures above 850°C in a CO2-deprived atmosphere, explained Dupont.
CaO can be used as a CO2 transfer material, by selectively removing CO2 from a polluted gas stream and releasing it later in a far more concentrated form into underground storage reservoirs.
One downside is that after multiple capture and regeneration cycles, the material’s capacity for capture decreases.
‘Despite this, CaO remains the most attractive of CO2 sorbents because of its abundance, and its potential for recuperating its initial CO2 capacity via a regeneration or modification process,’ explained Dupont.
If scientists can improve the life expectancy of CaO based materials then they could provide a low cost answer for carbon capture on a very large scale.
Before or after it goes up the chimney?
Current techniques for post-combustion filter out CO2 from a power plant’s flue gases as they travel up a chimney. The filter is a solvent, such as CaO, which absorbs the CO2, before being heated, releasing water vapour and leaving behind the CO2. The inherent advantage of post-combustion technology is that it has the potential to be retrofitted without drastically affecting process operations.
In pre-combustion, the CO2 is filtered out with a catalytic converter before the fossil fuel is burned and the CO2 is diluted by other flue gases. Both post- and pre-combustion have the potential to prevent 80-90 per cent of a power plant’s carbon emissions from entering the atmosphere.
Meanwhile, other completely different approaches include burning CO2 in pure oxygen (oxy-combustion), or turning CO2 into a mixture of carbon monoxide and hydrogen.
The key advantages of these processes are the potential for high CO2 separation efficiencies and the relative simplicity of the method, which potentially allows for retrofit.
Another more extreme idea is chemical looping, which involves taking oxygen to the fuel not as a gas, but bound to a metal such as iron or calcium. In principle this should make combustion more efficient.
But which techniques are closest to commercialisation? According to a report from Imperial College London’s Grantham Institute published in 2010 a number of technologies are at various stages of readiness. Post combustion ‘solvent scrubbing’ and pre combustion ‘integrated gasification’ were both considered being at a technology readiness level (TRL) of six on a scale of one to nine. Oxy-combustion was rated just behind at a TRL of five but is arguably less likely to receive as much attention because it demands a completely new plant to be built, while the others can be retrofitted.
Conclusion
Storage seems to be considerably easier than capture. The Intergovernmental Panel on Climate Change has estimated there is enough storage capacity for the next 200 years of global emissions and believe that 99 per cent of injected CO2 will remain trapped for 1000 years. What is more, storing CO2 underground can also lead to enhanced oil recovery from oil fields, which could boost oil outputs by 7-20 per cent.
Dr Paul Fennell from Imperial University’s Grantham Institute on Climate Change explained that the reason the CCS ball isn’t rolling with the momentum is requires in the UK the first government-backed competition - which was offering a £1bn prize to help commercialise CCS - didn’t generate a winner.
‘I suspect that it was about liabilities for the CO2 storage,’ said Fennell. ‘The companies would like to pass the liability onto the government because nobody wants to have a liability that is going to last for 50 - 100 years on their balance sheet.’
In reality, the progress of CCS is ultimately out of the hands of engineers and in the hands of politicians. If the UK wants to achieve its carbon emissions target of an 80 per cent carbon emission reduction by 2020 then this piece of technology, which could reduce coal power station carbon emissions by 80-90 per cent, is a no brainer.
UK Situation
The UK CCS Research Centre is a facility that has recently been set up by Research Councils UK to provide an overarching framework for CCS research in the UK.
The Pilot and Advanced CO2 Capture Technologies (PACT) has facilities in Sheffield, Cranfield and Edinburgh, looking at oxy-fuel combustion or post combustion technologies such as solvent scrubbing and solid looping cycles.
‘It’s a continuous process of development,’ said the Grantham Institute’s Paul Fennell. ‘R&D in the labs and trying to get them tested at a large scale.’
‘The real big prize will be to get the second round of the UK demonstration project up and off the ground. There’s a billion pounds on the table for the UK’s first major CCS demonstration.’
There are two sets of implications if we fail to act. One is for the UK and the other is for the world.
‘At a UK level, if CCS is developed elsewhere and the UK doesn’t play its part in the development then you’re looking at an industry that is about the same size as the oil industry is at the moment. It’s a trillion dollar industry that the UK would fail to secure it’s rightful share of,’ said Fennell. ‘We are currently world leading in the research areas and we punch well above our weight as it stands.’
‘At a global level, the consequences of failing to develop CCS technologies are that the cost of CO2-neutral electricity could sky-rocket because you’re relying on renewables and maybe nuclear,’ said Fennell. ‘The problem with these is that you can’t ramp them up and down in response to demand. Renewables switch on and off themselves without any controllability so what happens is you need to build hugely more renewables than you actually need to produce a particular power output.’ neutral electricity sky-rocket because you’re relying on renewables and maybe nuclear,’ said Fennell. ‘The problem with these is that you can’t ramp them up and down in response to demand. Renewables switch on and off themselves without any controllability so what happens is you need to build hugely more renewables than you actually need to produce a particular power output.’




Project to investigate hybrid approach to titanium manufacturing
What is this a hybrid of? Superplastic forming tends to be performed slowly as otherwise the behaviour is the hot creep that typifies hot...