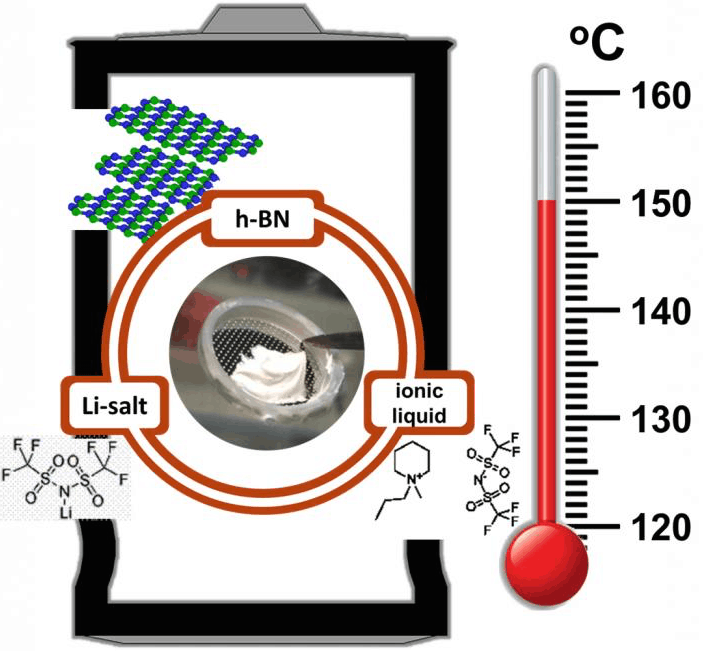
According to its developers at Rice University, Texas an essential part of the non-flammable, viscous composite is hexagonal boron nitride (h-BN).
The team led by materials scientist Pulickel Ajayan said batteries made with the composite functioned well in temperatures of 150 degrees Celsius (302 degrees Fahrenheit) for over a month with negligible loss of efficiency. Test batteries consistently operated from room temperature to 150 C, setting one of the widest temperature ranges ever reported for such devices, the researchers said.
"We tested our composite against benchmark electrodes and found that the batteries were stable for more than 600 cycles of charge and discharge at high temperatures," said lead author Marco-Túlio Rodrigues, a Rice graduate student.
The results were reported in Advanced Energy Materials.
In 2015 members of a Rice and Michigan-based Wayne State University team introduced an electrolyte made primarily of common bentonite clay that operated at 120 C. This year the team validated its theory that h-BN would serve the purpose even better.
Rodrigues said in a statement that batteries with the new electrolyte are geared more toward industrial and aerospace applications than mobile phones. In particular, oil and gas companies require robust batteries to power sensors on wellheads. "They put a lot of sensors around drill bits, which experience extreme temperatures," he said. "It's a real challenge to power these devices when they are thousands of feet downhole."
"At present, non-rechargeable batteries are heavily used for the majority of these applications, which pose practical limitations on changing batteries on each discharge and also for disposing their raw materials," said Rice alumnus and co-author Leela Mohana Reddy Arava, now an assistant professor of mechanical engineering at Wayne State.
Hexagonal boron nitride is not a conductor and is not known to be an ionic conductor, Rodrigues said. "So we didn't expect it to be any obvious help to battery performance. But we thought a material that is chemically and mechanically resistant, even at very high temperatures, might give some stability to the electrolyte layer."
He said boron nitride is a common component in ceramics for high-temperature applications. "It's fairly inert, so it shouldn't react with any chemicals, it won't expand or contract a lot and the temperature isn't a problem. That made it perfect."
The material also eliminates the need for conventional plastic or polymer separators, membranes that keep a battery's electrodes apart to prevent short circuits. "They tend to shrink or melt at high temperatures," said Rice postdoctoral researcher and co-author Hemtej Gullapalli.





Nanogenerator consumes CO2 to generate electricity
Whoopee, they've solved how to keep a light on but not a lot else.