Long thought to be impossible, imaging at such high resolution has allowed researchers to watch diseases taking hold in living cells, making possible huge strides in the development of new treatments.
Optical microscopy has long been thought limited because it was not possible to obtain clear images of anything smaller than half the wavelength of light, that is around 0.2micrometres. Although individual cells could be seen, the structures within them and even large protein molecules could not; viruses were also below this limit, which is knows as the Abbé Limit after microscopist Ernst Abbé, who came up with it in 1873.
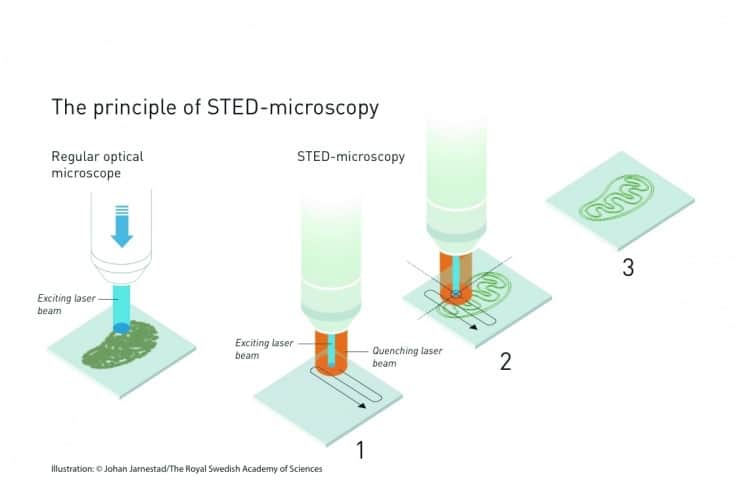
The first of the Nobel breakthroughs came from Prof Stefan Hell, a German microscopist working at Turku in Finland. Using a technique called stimulated emission, Hell devised a way to use lasers to both induce and quench — switch on and off — fluorescence in antibodies which could be coupled to DNA. Hell’s technique, called stimulated emission depletion, uses a laser beam with a nanoscale diameter to induce fluorescence, which is surrounded by a wider, ring-shaped quenching beam. Both beams scan over the sample, with the narrow central beam acting like a torch, illuminating a tiny fraction of the sample at any one time. The microscope’s processors combines the images from the scan with positional information of where the laser probe was when the image was obtained to give a combined image whose resolution matches the width of the inducing beam. Hell perfected the technique at the University of Göttingen in 2000.
The other development cake from two American researchers, WE Moerner and Eric Betzig. Moerner, working at the University of California in San Diego in 1997, discovered that single molecules of a green fluorescent protein that had been isolated from jellyfish could be made to fluoresce, then turned off and on again, using a specific wavelength of light.
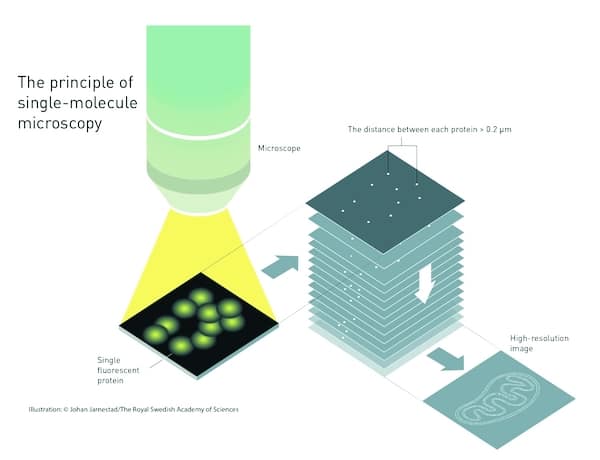
Betzig, following Moerner’s work closely, realised in 2006 that by coupling this protein to the membrane surrounding a specific part of a cell, a light pulse at the right wavelength would cause the fluorescent molecules to glow; but if the light used was weak, only some of the molecules would fluoresce, and most of them would be far enough apart that a normal light microscope could resolve them. After a short time the fluorescence would die away, then the weak pulse would be repeated to make more molecules fluoresce. Repeating this many times and imaging each group of glowing points, then superimposing these images, gave a composite image with resolution well below the Abbé Limit.
The three Nobel winners are still active in the field. Hell is working on the mechanism of brain cell signalling across synapses; Moerner on the fata; genetic degenerative condition Huntington’s disease; and Betzig on cell divisions inside developing embryos.





Red Bull makes hydrogen fuel cell play with AVL
Formula 1 is an anachronistic anomaly where its only cutting edge is in engine development. The rules prohibit any real innovation and there would be...