As a milestone in medical history the invention of the heart-lung bypass machine is up there with the discovery of antibiotics or the mapping of the human genome.
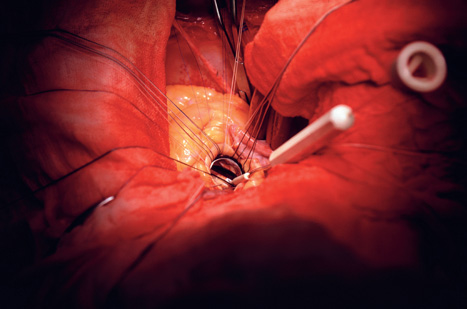
First used in 1953 by US surgeon John Gibbon, the machine ushered in a new era of open-heart surgery. Procedures once considered impossible became routine. And almost 60 years later it is firmly established as the critical tool in a heart surgeon’s armoury.
But despite its pedigree, the technology is not without risks. Bypass machines can degrade the quality of a patient’s blood and potentially hamper recovery from surgery. While more critically, the process of reperfusion - when the surgeon restarts the heart and reintroduces blood to the tissue - has been linked with damage to the heart, strokes and neurological problems.
In an effort to reduce these risks, heart surgeons are increasingly looking at ways of operating on the heart without resorting to bypass machines. While the list of procedures where this is feasible is currently short, a new generation of robotic devices able to compensate for organ movement could soon enable specialists to perform a variety of operations on a patient’s still-beating heart.
One such system is being developed by a team of engineers at the German Aerospace Center (DLR), which is applying its expertise in developing lightweight tele-operated space robots to the more down-to-earth demands of the operating theatre.
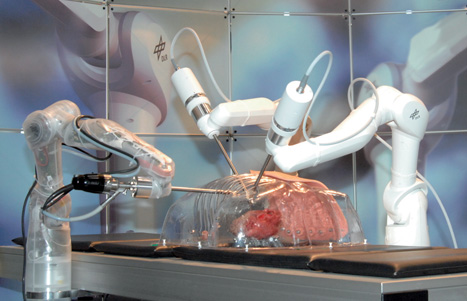
Developed to enable surgeons to perform minimally invasive surgery, the group’s prototype Mirosurge system consists of three robot arms: two carrying specialised surgical instruments and the third equipped with a high-definition stereo endoscope that relays images back to the surgeon via a 3D display.
Although the robot hasn’t yet entered commercial use, its developers claim it has some compelling advantages over existing tele-operated systems - not least its use of force feedback technology that enables surgeons to actually feel the force measured at the tip of its surgical instruments.
But most intriguingly, the group is now equipping the robot with technology that could enable surgeons to operate on an apparently motionless heart that is, in fact, still beating.
According to Martin Groeger, one of the researchers on the team, this motion compensation system has a number of key elements. He said: ’We track the motion of the beating heart using an endoscopic camera and then we feed it back to the instruments so that they will be able to move in accordance to the motion of the heart beat.’ Advanced algorithms are then used to stabilise the images acquired by the camera before they appear on the surgeon’s display screen. Thus, while the heart is still beating, and the surgical instruments moving in relation to this beat, the surgeon using the system views a stabilised and effectively motionless video feed.
In such a highly intensive process, there are inevitably time delays while the system analyses the images and controls the robot, and a series of specially developed prediction algorithms help compensate for these time delays. ’As you can imagine it takes some time until motion information is gained from the images and delivered to the robotic system,’ said Groeger. ’To achieve synchronous motion we need to compensate for the latencies that occur in the system.’ This prediction component also helps the system compensate for short-term occlusions if, for instance, the cutting instrument is temporarily obscuring the view of the camera. ’We cannot allow for any failures during surgery,’ Groeger added. ’This is why we combine our motion tracking and imaging approach with a prediction component that is able to detect disturbances and also to compensate for short-time occlusions by instruments.’
Although the system is several years away from being used in an operating theatre, Groeger is confident that it could one day make a major difference. ’Adding a motion compensation component to the system makes it possible for surgeons to operate on a beating heart. In this way, you don’t need the heart-lung machine and you avoid complications later on for the patient.’ He added that the system could also be used to operate on other moving organs, such as the lungs.
In the meantime, the DLR group is continuing to refine and test the system. Currently, these tests are performed on an artificial heart simulator also developed by the group. The next stage is to move on to animal testing.
Although the DLR group’s work is at a relatively advanced stage, a number of other teams around the world are investigating the motion compensation challenge.
Here in the UK, at Imperial College London, former health minister and robotic surgery pioneer Lord Ara Darzi has been working with his colleague Prof Guang-Zhong Yang on software that makes a beating heart appear still to a surgeon. The system uses cameras to track how a surgeon’s eyes refocus on ’fixation points’ as the heart expands and contracts. Using the changes to the eye as focus shifts, scientists can determine ’depth perception’ and precise dimensions of the moving heart via a robotic laparoscope, with the blade then replicating the movements as it carries out an incision.

Meanwhile, in the US, engineers at Harvard University and Children’s Hospital Boston are testing a similar system that uses 3D ultrasound images to predict and compensate for the motion of the heart so that the surgeon can work on a patient’s beating heart. Designed for mitral-valve surgery, which is apparently performed on around 50,000 patients a year in the US, the procedure uses a hollow needle to put small anchors into the heart and affix them to the tissue around the mitral valve. The anchors can then be pulled together by a suture wire to decrease the size of the valve opening.
To keep track of the heart during the process of affixing the anchors, the team uses 3D ultrasound coupled with prediction software to adjust the tip of the handheld surgical tool. It’s hoped that the procedure could one day replace traditional treatments, which reduce the size of the mitral valve by placing a stiff ring around the valve and suturing it in place by hand.
Motion compensation technology is also helping drive the development of radiotherapy
Most recent developments in radiotherapy have focused on improving targeting - and so reducing damage to surrounding tissue - through techniques that enable clinicians to shape radiation beams to closely approximate the shape of the tumour. However, a number of groups are now taking this targeted approach to a whole new level by developing systems able to blitz moving organs with precisely directed bursts of radiation. For instance, as previously reported in The Engineer (14 February, p33) US company Calypso Medical has developed a system that tracks the motion of an organ while X-rays are delivered to it through the use of implanted electro-magnetic transponders.
Meanwhile, rival US organisation Accuray has developed an alternative approach to treating moving organs with its Cyberknife system, which uses a highly agile robotic arm to blast hard-to-reach tumours from hundreds of different angles.
During treatment on the machine, a suite of advanced image-guidance techniques ensure the high-energy beams stay locked on their targets. Stereoscopic imagers mounted in the floor and ceiling track the anatomy and trigger miniscule adjustments to the system, while, for abdominal and thoracic treatments, an arrangement of X-ray cameras and a vest equipped with LEDs are used to monitor the patient’s breathing and reposition the radiotherapy beam accordingly.




Swiss geoengineering start-up targets methane removal
No mention whatsoever about the effect of increased methane levels/iron chloride in the ocean on the pH and chemical properties of the ocean - are we...