The nanotubes can be tuned to have a diameter of between five and 10nm, stacking together to form tubes up to 100nm long. According to the researchers they have many potential uses, including delivering cancer drugs into the cells of patients as well as for the desalination of seawater.
"Creating uniform structures in high yield is a goal in nanotechnology," said Ron Zuckermann, director of the Biological Nanostructures Facility in Berkeley Lab's Molecular Foundry.
"For example, if you can control the diameter of nanotubes, and the chemical groups exposed in their interior, then you can control what goes through, which could lead to new filtration and desalination technologies, to name a few examples."
Published in the journal Proceedings of the National Academy of Sciences, the work is the latest effort to build nanostructures that mimic the complexity of natural proteins but are made of durable materials. The researchers focused on a polymer from the peptoid family called a diblock copolypeptoid. This acts like a synthetic equivalent to the peptides that form naturally in proteins.
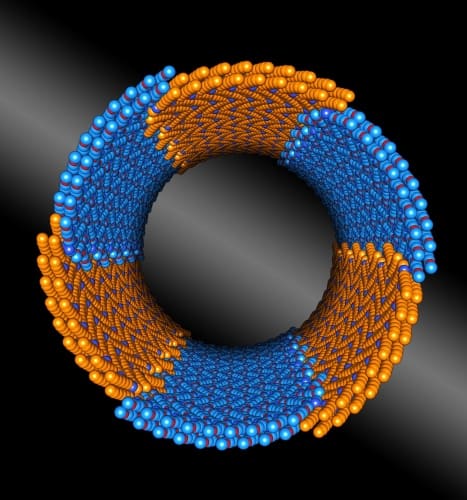
Diblock copolypeptoids are composed of two peptoid blocks, one of which is hydrophobic while the other is hydrophilic. The scientists discovered both blocks crystallise when they meet in water, and form rings consisting of two to three individual peptoids. These rings then form hollow nanotubes.
The peptoids are thought to arrange themselves in a brick-like pattern, with hydrophobic blocks lining up with other hydrophobic blocks, and the same for hydrophilic blocks. On top of this, the nanotubes assemble themselves without the usual construction aids, such as electrostatic interactions or hydrogen bond networks.
"You wouldn't expect something as intricate as this could be created without these crutches, but it turns out the chemical interactions that hold the nanotubes together are very simple,” said Zimmerman.
“What's special here is that the two peptoid blocks are chemically distinct, yet almost exactly the same size, which allows the chains to pack together in a very regular way. These insights could help us design useful nanotubes and other structures that are rugged and tunable, and which have uniform structures."




Glasgow trial explores AR cues for autonomous road safety
They've ploughed into a few vulnerable road users in the past. Making that less likely will make it spectacularly easy to stop the traffic for...