A method to treat chronic nerve pain at its source with the body’s own painkillers with no side effects could also be used to deliver other drugs to the brain and to other parts of the body, according to its developers at Linköping University in Sweden. The device, which has so far only been tested on animals in accordance with medical device regulations, works by preventing pain signals from reaching the brain at all.
In a paper in the journal Science Advance — an open-access sister publication to Science — the team, led by Prof Magnus Berggren, explains that chronic pain is suffered by some 30 per cent of the population, with seven per cent suffering from neuropathic pain, where damaged or misfiring nerves leave the sufferer in constant misery which, furthermore, is difficult to treat. Standard therapies using painkillers tends to be systemic — that is, the drug is delivered to the whole body, with only a fraction of the dosage actually affecting the pain, and the rest can cause side-effects elsewhere. Targeting pain precisely is notoriously difficult.
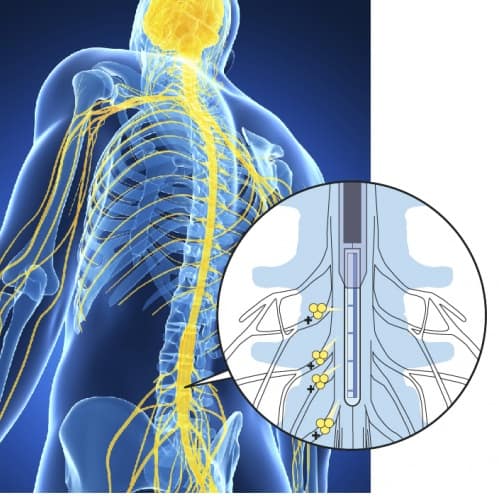
The Swedish team’s approach to this is to implant a small device in the spinal column that delivers therapy to the precise point where the affected nerves join the spinal cord. The device is an ion pump — that is, it works by translating electrical signals into pulses of charged molecules — and the compound the team used it to release is a neurotransmitter called GABA (g-aminobutyic acid) whose function is to inhibit signals travelling along nerves. This has the effect of stopping the brain from ever receiving the pain impulse — the nerve is still damaged, but the brain does not know it.
The device, called an organic electronic ion pump (OEIP), was developed by another team led by Prof Berggren in 2009. It uses organic polymers that are capable of conducting electricity, based on the polymer polyethtlene terephthalate (PET), most familiar for its use in plastic bottles but which also has a long history of usage in medical implants. A blend of conducting polymers was printed onto a PET substrate to a reservoir that supplied four delivery channels or fingers; this pattern was treated so that it could only conduct positively-charged ions. Furthermore, the channels were designed to vary the rate of delivery to each of the four fingers. Applying a current to the OEIP forces ions to flow, completing the electrical circuit; in this case, the GABA ions spread around the nerve junction where the fingers are attached, forming a thin cloud, but not diffusing out into the rest of the body.
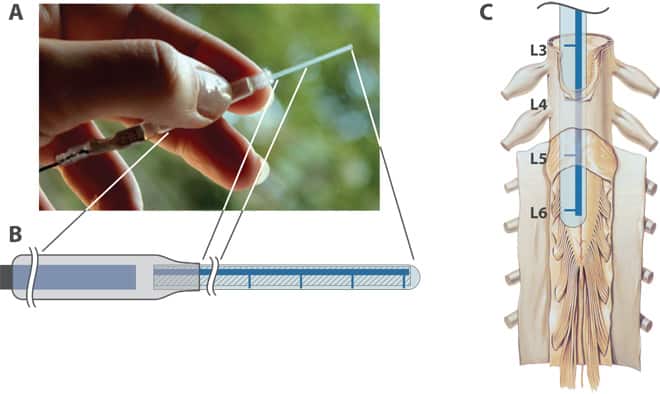
Working with rats at the Karolinska Institute, an institution that specialises in highly-regulated animal research, the team tailored the device to treat specific nerve damage, and administered GABA while the rats were awake and free to move. They found that GABA reduced the rats’ pain symptoms significantly, compared with a control test where the OEIP delivered only hydrogen ions. “What’s unique is that we’re using organic electronics to send the body’s own chemical signals,” said Assistant Prof Daniel Simon, who worked on the original development of the OEIP and was in charge of the tests. “The organic materials are easily accepted by the body, and they communicate just as in biology – with charged ions.”
The device compares favourably with existing treatments, the team said, targeting the pain better and with more controllability. For human application, the delivery fingers would have to be longer, thicker and softer than the test device, but can be tailored to be positioned at the specific nerve junctions affected and to deliver GABA or any other ionic pharmaceutical agent at a defined rate; the device is refillable as a routine procedure, they add.
The team sees this development as part of a long-term vision of treating conditions caused by cell signalling, of which there are many, by what they call ‘iontronics’. ‘Effecting therapy by organic electronics in awake, freely-moving animals is an important step towards this goal,’ they say in the paper. Successful animal trials pave the way for future human trials




Red Bull makes hydrogen fuel cell play with AVL
Many a true word spoken in jest. "<i><b>Surely EVs are the best solution for motor sports</b></i>?" Naturally, two electric motors demonstrably...