A new device for removing liver tumours with virtually no blood loss has been successfully used for the first time in the
The Habib 4X resection device is named after its inventor Professor Nagy Habib, Professor of Hepato-Biliary Surgery at Imperial College London and chief of service for gastrointestinal surgery at Hammersmith Hospitals NHS Trust in west
The new device uses radiofrequency energy to 'seal' tissue around a tumour site, allowing the tumour to be removed while preventing blood loss and other complications. The device has enabled surgeons to operate where previously it would have been too risky.
After developing the technology, Professor Habib formed an
The Habib 4X received approval from the US Food and Drug administration in August, and the first operation using the Habib 4X took place at the City of Hope National Medical Center in
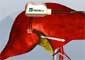
The Habib 4X works by delivering high-energy radio waves through a hand held device consisting of four electrodes into tissue around the tumour. They heat cells causing them to dehydrate and thus form a seal. The tumour is removed with a scalpel, with virtually no blood loss, and without the use of staples, glue, ties, and sutures.
Before use of the device in the
Over 100 patients have been operated on with the new device since October 2004, and none have died or suffered serious illness after the operation. The average hospital stay has been reduced from two weeks to eight days. When patients were followed up over a period of between two and 20 months, tumours had not returned in any of them.




Glasgow trial explores AR cues for autonomous road safety
They've ploughed into a few vulnerable road users in the past. Making that less likely will make it spectacularly easy to stop the traffic for...