In the early days of the Covid-19 pandemic, an emergency call was put out to UK industry for assistance of all kinds. The nature of the virus meant that one of the most pressing needs was for ventilators, complex biomedical machines that assist gravely ill patients with breathing.
As hospitals across the UK started seeing increasing numbers admitted with breathing difficulties, companies from right across the manufacturing spectrum came together on multiple ventilator projects. Rather than single out an individual effort, The Engineer’s C2I award for Covid Response recognises the work of several collaborations that rose to this challenge.
C2I 2020Category: COVID-19 response
Winner: Zephyr Plus Ventilator
Partners: Babcock International with Raytheon, Kohler Mira Limited, Kinneir Dufort, Future Advanced Manufacture, PDD, Amtek Precision, Plexus
Despite having no significant experience in the medical sector, Babcock brought together specialists from around the UK and Germany to create not only a ventilator solution, but also two fully functioning manufacturing facilities within 87 days.
The first working prototype was up and running just five days after the project was initiated, as well as a fully developed manufacturing system concept. According to Babcock, one of the most complex elements of the ventilator was the control systems design, responsible for regulating patient breathing. This part of the device not only had to meet extremely close tolerances, it had to endure robust testing to ensure its safety and reliability.
With more than 50 partners and suppliers feeding into the project, communication and trust were essential. A robust governance framework was put in place in order to empower project leaders and ensure that accountability, fairness and transparency remained at the heart of the collaboration. Seven days a week, a single core scrum meeting was held which then drove the agenda for the individual sub teams. Communication was facilitated via a central repository on SharePoint and a variety of conferencing software, with WhatsApp even being used for ad hoc updates.
The end result was a low-cost, high-quality ventilator more akin to the type of equipment found in ICUs rather than a makeshift device for use in emergency situations. Although the Zephyr Plus did not come to market, it remains ready to do so at any time in the future, alongside a manufacturing pipeline that can turn out 10,000 units in just a six-week window.
CLICK FOR COVID-19 RESPONSE SHOTLISTED ENTRIES
C2I 2020Category: COVID-19 response
Winner: OxVent
Partners: Oxvent Ltd with University of Oxford, King’s College London and Smith+Nephew
Within a week of the UK Ventilator Challenge being announced, an early incarnation of the OxVent team was demonstrating a low-cost prototype to the Cabinet Office. Though it ultimately transpired that the clinical demand for ventilators was lower than initially feared, the collaboration has produced a brand new medical device, ready to be deployed in emergency situations.
According to OxVent, most of the ventilator’s 100 or so components are available off-the-shelf and it features just a single moving part. Based on the pneumatic “bag-in-a-bottle” principle, the device has a proportional solenoid valve which controls injection of air at 4 bar into a sealed box compressing a standard bag valve mask inside, displacing an equal volume of air which is used to ventilate the patient.
https://vimeo.com/507175364
Lung injury due to overpressure is prevented by both hardware and software controls, and additional protection is provided by a full set of alarms. There are two modes of operation: mandatory ventilation and assist control ventilation, whereby attempts by the patient to breathe spontaneously are detected and trigger the delivery of a breath at a pre-set volume. As a volume control ventilator, OxVent also has much lower oxygen requirements than standard pressure control ventilators and high-flow oxygen therapy, reducing demand on the oxygen supply of hospitals.
A single manufacturing line at Smith+Nephew’s Hull site was set up with production capability of 5,000 units per week at cost price of around £1,000 per unit. Demand in the UK meant that this facility was never called into action, but OxVent is exploring opportunities to supply its device to low and middle income countries (LMICs), where demand for mechanical ventilation is expected to grow regardless of Covid, and where a shortage of the right equipment can be a matter of life and death.
C2I 2020Category: COVID-19 response
Winner: Emergency Ventilator for Covid-19
Partners: Jenton International Limited with B&R Industrial Automation Limited, Festo Limited, Dero Fabrication Limited and Shearmans Limited
Jenton International is a Hampshire-based manufacturing SME that employs around 25 people. Founded in 1973, today it specialises in automation systems for the food industry. Despite having no previous medical expertise, it felt compelled to answer the government’s call to action, relying on the company’s knowledge in process automation to underpin the creation of an emergency ventilation device.
https://vimeo.com/498027821/e71302ca16
According to Jenton, both areas require machines with complex control algorithms to ensure a high level of repeatability and accuracy under a range of changeable conditions. For the ventilator, this meant being able to supply a required volume and ratio of air or oxygen to a patient in a set time, but with a varying back pressure and limitations on maximum and minimum criteria. In addition, the system had to be fail-safe and to be used by medically trained professionals in a manner that they would expect from standard commercially available systems.
Setting aside commercial rivalries, the collaborating partners produced a working prototype within three weeks, sourcing components from outside existing ventilator supply chains, as all of the Ventilator Challenge participants were required to do. Lower than predicted demand meant the device never made it into production, but Jenton recognises that this in itself was a positive outcome, and the collaboration with like-minded engineering companies in the name of a worthy cause was its own reward.
C2I 2020Category: COVID-19 response
Winner: The InVicto Ventilator:
Partners: JFD with Frazer-Nash Consultancy, Angus 3D Solutions, MDU, Narayana Health and Innovhealth
Utilising 3D printing and advanced computational fluid dynamics (CFD), the JFD-led consortium developed a novel type of non-invasive ventilator, essentially carving out an entirely new category of medical device.
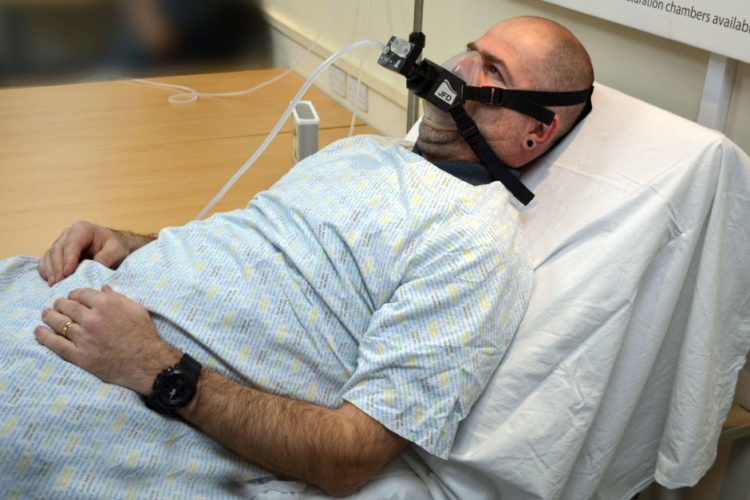
Traditional ICU ventilators require the intubation of a patient, whereby an endotracheal tube is passed down into the lungs. It’s a specialised technique that requires no small amount of clinical skill - as well as the sedation of the patient – but provides close therapeutic control over the breathing cycle. Less invasive techniques such as CPAP (Continuous Positive Airway Pressure) can assist with shortness of breath. However, CPAP requires the patient to be breathing in order to be effective and is of limited use in severe Covid cases.
The InVicto Ventilator seeks to combine the advantages of both, providing full ventilation to patients who cannot breathe independently, yet avoiding the need for invasive intubation. JFD first worked with Angus 3D solutions to rapidly create a number of prototype polymeric ventilators. Later, the design was refined in partnership with Frazer-Nash Consultancy, which developed a CFD model to simulate the flow of gases through the ventilator fluid logic element during the entire breathing cycle. Due to InVicto’s flexible design, the device is suitable in a range of scenarios, providing full ventilation support with patient monitoring, breathing gas and oxygen management and alarms.




Swiss geoengineering start-up targets methane removal
No mention whatsoever about the effect of increased methane levels/iron chloride in the ocean on the pH and chemical properties of the ocean - are we...