
The research conducted at the McKelvey School of Engineering at Washington University in St. Louis has been published in Cell Reports Physical Science.
Metal-organic framework materials show promise for fuel cells
The research team, led by Vijay Ramani, the Roma B. and Raymond H. Wittcoff Distinguished University Professor, has pioneered a reactant: identifying an optimal range of flow rates, flow field architectures and residence times that enable high power operation. This approach is said to address key challenges in DBFCs, namely proper fuel and oxidant distribution and the mitigation of parasitic reactions.
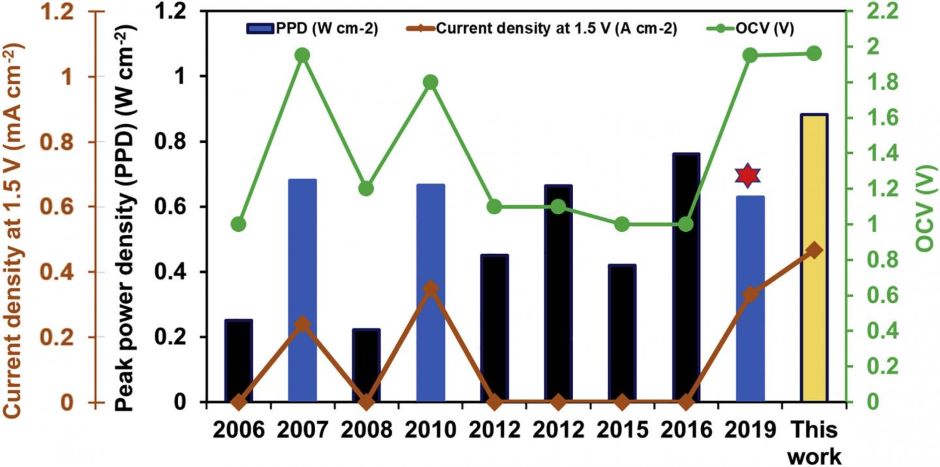
The team has demonstrated also a single-cell operating voltage of 1.4 or greater, double that obtained in conventional hydrogen fuel cells, with peak powers approaching 1W/cm2.
Doubling the voltage would allow for a smaller, lighter, more efficient fuel cell design, leading to gravimetric and volumetric advantages when assembling multiple cells into a stack for commercial use. Their approach is said to be broadly applicable to other classes of liquid/liquid fuel cells.
"The reactant-transport engineering approach provides an elegant and facile way to significantly boost the performance of these fuel cells while still using existing components," Ramani said in a statement. "By following our guidelines, even current, commercially deployed liquid fuel cells can see gains in performance."
The key to improving any existing fuel cell technology is reducing or eliminating side reactions. Most efforts to achieve this goal involve developing new catalysts that face challenges in terms of adoption and field deployment.
"Fuel cell manufacturers are typically reluctant to spend significant capital or effort to adopt a new material," said Shrihari Sankarasubramanian, a senior staff research scientist on Ramani's team. "But achieving the same or better improvement with their existing hardware and components is a game changer."
"Hydrogen bubbles formed on the surface of the catalyst have long been a problem for direct sodium borohydride fuel cells, and it can be minimised by the rational design of the flow field," said Zhongyang Wang, a former member of Ramani's lab who is now at the Pritzker School of Molecular Engineering at the University of Chicago. "With the development of this reactant-transport approach, we are on the path to scale-up and deployment."




Red Bull makes hydrogen fuel cell play with AVL
Formula 1 is an anachronistic anomaly where its only cutting edge is in engine development. The rules prohibit any real innovation and there would be...