Angioplasty opens blocked arteries and can involve placing a metal stent to reinforce arterial walls and prevent them from collapsing once the blockage is removed. According to NC State, the stent's placement usually causes some injury to the blood vessel wall, which stimulates smooth muscle cells to proliferate and migrate to the site to repair the injury. This leads to restenosis, which is a re-narrowing of the blood vessel previously opened by angioplasty.
Biodegradable stent helps children with LTS to breathe more easily
Smart stent feels the squeeze to alert doctors and keep patients safe
"The inflammatory response that stents cause can decrease their benefit," Ke Cheng, corresponding author of the research, said in a statement. "Ideally, if we could stop smooth muscle cells from over-reacting and proliferating, but recruit endothelial cells to cover the stent, it would mitigate the inflammatory response and prevent restenosis."
Current drug-eluting stents are coated with drugs that discourage cell proliferation, but these anti-proliferative drugs also delay stent coverage by endothelial cells, which are the cells healthcare providers want to coat the stent with.
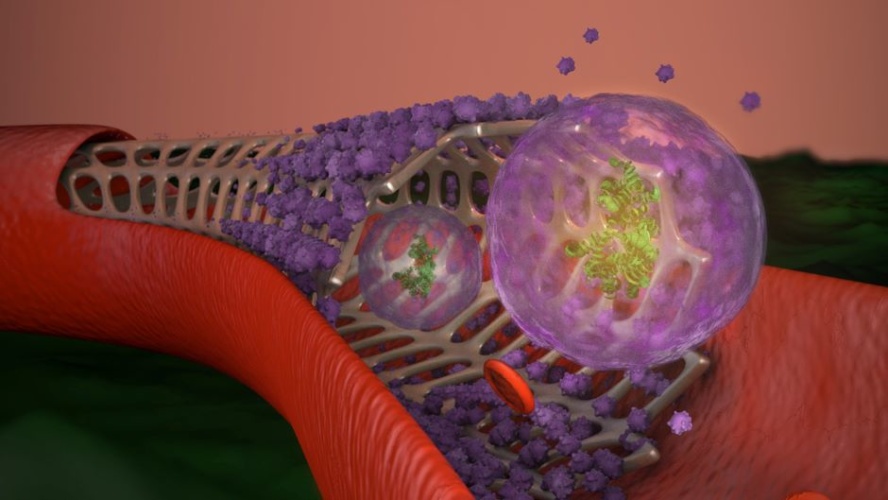
Cheng and his team are said to have developed a stent coating composed of exosomes derived from mesenchymal stem cells. Exosomes are nano-sized sacs secreted by most cell types. The idea behind the coating was two-fold: first, since the exosomes are composed of materials not much different from cell membranes they 'camouflage' the stent to trick smooth muscle cells and the body's immune system. Second, the exosomes promote coverage of the stent by endothelial cells and, in the case of injury, travel downstream to the site to promote tissue repair.
To prevent premature depletion of the therapy, the stent releases exosomes when it encounters reactive oxygen species (ROS) - which are more prevalent during an inflammatory response.
"Think of it as a smart release function for the exosomes," Cheng said. "Ischemic reperfusion injuries, which occur when blood flow is diminished and then reestablished, create a lot of ROS. Let's say the heart is damaged by ischemia. The enhanced ROS will trigger the release of the exosomes on the stent, and regenerative therapy will travel through the blood vessel to the site of the injury."
The research team performed in vitro testing to ensure biocompatibility and test the release mechanism and found that in the presence of ROS, the exosomes released up to 60 per cent of their secretions within 48 hours post-injury.
In a rat model of ischemic injury, the researchers compared their exosome-eluting stent (EES) to a bare metal stent (BMS) and a drug-eluting stent (DES). They found that in comparison to the BMS, their stent performed better in both decreasing stenosis and promoting endothelial coverage. While the DES performed similarly to the EES in preventing restenosis, the EES was less injurious to the vessel wall and had better endothelial coverage overall. In addition, the exosomes released from EES promoted muscle regeneration in rats with hind limb ischemia. The researchers plan to test the stent in a large animal model with an eye toward eventual clinical trials.
"This bioactive stent promotes vascular healing and ischemic repair, and a patient wouldn't need additional procedures for regenerative therapy after the stent is in place," said Cheng, who is the Randall B. Terry Jr. Distinguished Professor in Regenerative Medicine at NC State and a professor in the NC State/UNC-Chapel Hill Joint Department of Biomedical Engineering. "The stent is the perfect carrier for exosomes, and the exosomes make the stent safer and more potent in tissue repair."
The team’s research has been published in Nature Biomedical Engineering.




Red Bull makes hydrogen fuel cell play with AVL
Formula 1 is an anachronistic anomaly where its only cutting edge is in engine development. The rules prohibit any real innovation and there would be...