The technology, developed by Prof Guntae Kim at Ulsan National Institute of Science and Technology (UNIST) in collaboration with material scientists and engineers at the Georgia Institute of Technology, depends on a well-understood phenomenon: the dissolution of carbon dioxide into water to produce an acidic solution, which occurs in nature when carbon dioxide dissolves in the oceans.
Prof Kim and the team realised that this could be used to induce an electrochemical reaction. The creation of an acidic solution increases the number of protons in the water, each of which can attract an electron, and this implies that a battery system can be created.
"Carbon capture, utilisation, and sequestration (CCUS) technologies have recently received a great deal of attention for providing a pathway in dealing with global climate change," says Prof Kim. "The key to that technology is the easy conversion of chemically stable CO2 molecules to other materials." He adds, "Our new system has solved this problem with CO2 dissolution mechanism."
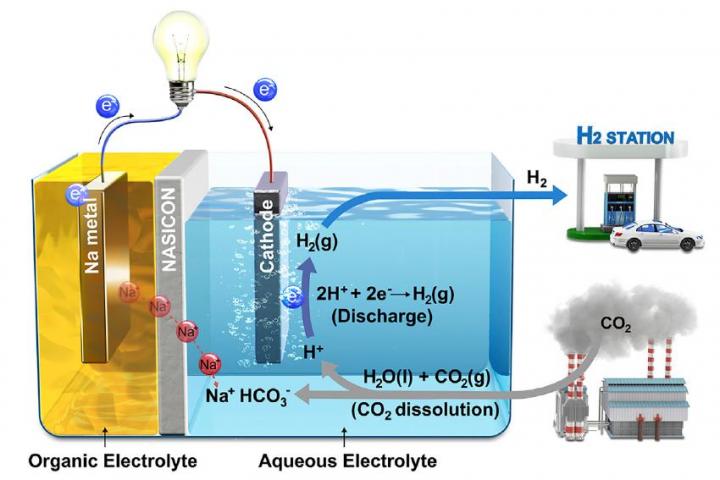
The fuel cell system consists of a sodium metal anode submerged in an organic electrolyte, a separation membrane consisting of a sodium super ionic conductor (NASICON) ceramic, and a catalytic cathode (the researchers used platinum) in an aqueous electrolyte, which could be distilled water, seawater or a sodium hydroxide solution. The researchers explain in iScience how injecting carbon monoxide into the water triggers a reaction, with gaseous hydrogen liberated at the cathode – which can then be used in conventional fuel cells – and current flowing in an external circuit. Sodium ions are liberated from the anode, pass through the membrane and recombine with the hydrogen carbonate ions formed by the dissolution of carbon dioxide. In the system’s current form, the conversion efficiency of CO2 is 50 per cent
The “hybrid Na-CO2 cell” continues to produce electricity and hydrogen and does not regenerate carbon dioxide during charging, the team says. The system has been tested over more than a thousand hours with no damage to the electrodes. "This hybrid Na- CO2cell, which adopts efficient CCUS technologies, not only utilises CO2 as the resource for generating electrical energy but also produces the clean energy source, hydrogen," said Jeongwon Kim, electrical engineer at Unist and co-first author for the research.
"This research will lead to more derived research and will be able to produce H2 and electricity more effectively when electrolytes, separator, system design, and electrocatalysts are improved."




Red Bull makes hydrogen fuel cell play with AVL
Formula 1 is an anachronistic anomaly where its only cutting edge is in engine development. The rules prohibit any real innovation and there would be...