Scientists at Rice University have used graphene quantum dots combined with nitrogen atoms to transform CO2 into liquid fuel.
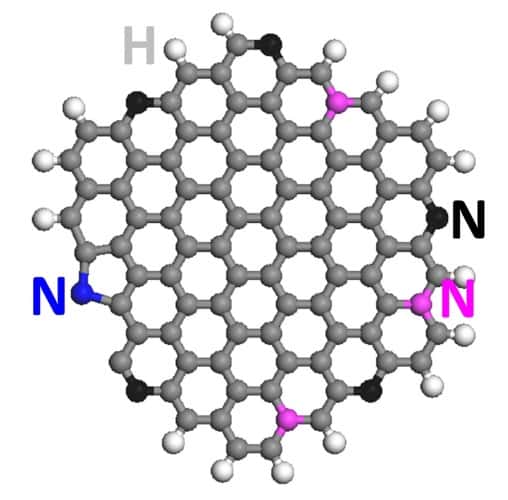
Described in the journal Nature Communications, the nitrogen-doped graphene quantum dots (NGQDs) act as an electrocatalyst, converting the greenhouse gas into small batches of ethylene and ethanol. Although the researchers say they don’t fully understand the mechanism at work, the NGQDs are as efficient a catalyst as copper, which is also being tested to turn CO2 into complex hydrocarbons. Furthermore, the quantum dots also retain their catalytic activity for a long time.
“It is surprising because people have tried all different kinds of catalysts, and there are only a few real choices such as copper,” said Rice materials scientist Pulickel Ajayan, who led the research.
“I think what we found is fundamentally interesting, because it provides an efficient pathway to screen new types of catalysts to convert carbon dioxide to higher-value products.”
The quantum dots are atom-thick sheets of carbon which have been split into particles about a nanometre thick and just a few nanometres wide. Adding nitrogen atoms enables various chemical reactions to take place when an electric current is applied and a feedstock like carbon dioxide is introduced. In lab tests, NGQDs were able to reduce carbon dioxide by up to 90 per cent, converting 45 per cent into either ethylene or alcohol.
“Our findings suggest that the pyridinic nitrogen (a basic organic compound) sitting at the edge of graphene quantum dots leads the catalytic conversion of carbon dioxide to hydrocarbons,” said Rice postdoctoral researcher Jingjie Wu, co-lead author of the paper.
“The next task is further increasing nitrogen concentration to help increase the yield of hydrocarbons.”
While the electrocatalysis can easily be performed in the lab, industry generally uses scalable thermal catalysis to produce fuels and chemicals. As a result, said Ajayan, the process is unlikely to be adopted in the near future for commercial purposes.




Swiss geoengineering start-up targets methane removal
No mention whatsoever about the effect of increased methane levels/iron chloride in the ocean on the pH and chemical properties of the ocean - are we...