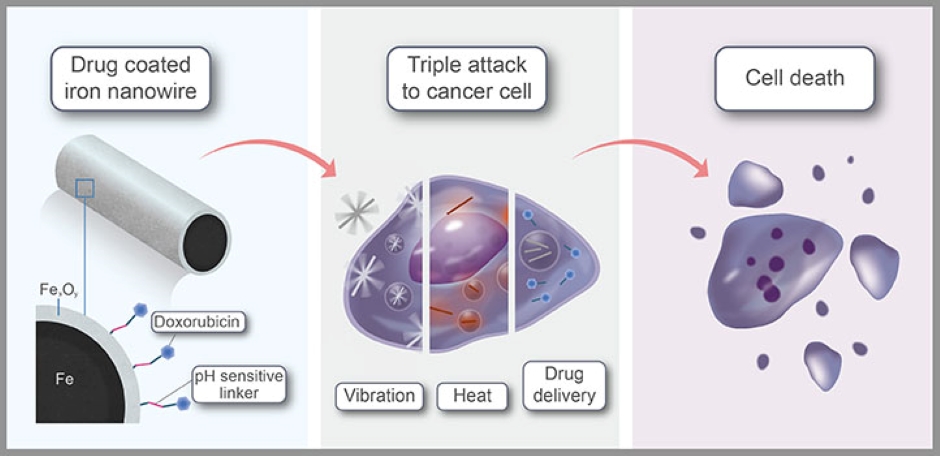
This is the claim of researchers at KAUST, Saudi Arabia and CIC biomaGUNE, Spain whose iron nanowires are said to release their drug cargo inside cancer cells while penetrating the cell's membrane and delivering a blast of heat. While the combination therapy maximises cancer cell death, its highly targeted nature should minimise side effects. The findings are published in ACS Applied Materials & Interfaces.
Magnetic surgical cement guides chemo drug delivery
Nano particle drug delivery technology could offer hope for difficult-to-treat brain cancer
Iron was the obvious material to make the nanowires, said Jürgen Kosel, who leads the group at KAUST, which includes Jasmeen Merzaban and Boon Ooi, and who co-led the work with researchers from CIC biomaGUNE in San Sebastian.
"Iron, in molecular form, is a native material in our bodies, essential for oxygen transport," Kosel said in a statement. The nanowires comprise an iron core, coated with an iron oxide shell. "Iron-oxide-based nanomaterials have been approved by regulatory bodies for use in magnetic resonance imaging and as a dietary supplement in cases of nutrition deficiency," he said.
In addition to their biocompatibility, the magnetic properties of iron-based materials are a key benefit.
"Using harmless magnetic fields, we can transport them; concentrate them in the desired area; rotate or make them vibrate, such as we did in this study; and even detect them through magnetic resonance imaging," said Aldo Martínez-Banderas, a member of Kosel's team. Applying low-power magnetic fields, the team agitated the nanowires in a way that opened the membrane of target cells, inducing cell death.
The additional advantage is that core-shell inanowires strongly absorb near-infrared light, heating up as they do so. Because light at this wavelength can penetrate far into the body, the iron nanowires could be heated using lasers directed at the tumour site.
"The core-shell nanowires showed an extremely high photothermal conversion efficiency of more than 80 per cent, which translated into a large intracellular heat dose," Martínez-Banderas said.
The anticancer drug doxorubicin was attached to the nanowires via pH-sensitive linkers. As the tumour environment is typically more acidic than healthy tissue, the linker selectively degraded in or near tumour cells, releasing the drug where it is needed. "The combination of treatment resulted in nearly complete cancer cell ablation and was more effective than individual treatments or the anticancer drug alone," Martínez-Banderas said.
"Taken together, the capabilities of iron-based nanomaterials make them very promising for the creation of biomedical nanorobots, which could revolutionise healthcare," Kosel said. "While this might seem futuristic, the developments are well on their way."




Swiss geoengineering start-up targets methane removal
No mention whatsoever about the effect of increased methane levels/iron chloride in the ocean on the pH and chemical properties of the ocean - are we...