(Credit: Harvard SEAS)
By heating the tips of the pyramids to 300°C with nanosecond laser pulses, the team created tiny bubbles that could push into cell membranes without causing damage. The brief opening of cell pores allowed surrounding molecules to diffuse into the cell. Published in the journal ACS Nano, the work has wide-ranging implications for healthcare.
"Being able to effectively deliver large and diverse cargos directly into cells will transform biomedical research," said first author Nabiha Saklayen, a PhD candidate from the Harvard John A Paulson School of Engineering and Applied Sciences (SEAS).
"However, no current single delivery system can do all the things you need to do at once. Intracellular delivery systems need to be highly efficient, scalable, and cost-effective while at the same time able to carry diverse cargo and deliver it to specific cells on a surface without damage. It's a really big challenge."
Saklayen and her colleagues had previously demonstrated that the gold microstructures are very good at focusing laser energy into electromagnetic hotspots. Using a fabrication method called template stripping, the team was able to populate a surface the size of a US quarter with 10 million of the tiny pyramids.
"The beautiful thing about this fabrication process is how simple it is," said Marinna Madrid, Harvard SEAS PhD candidate and co-author of the paper. "Template-stripping allows you to reuse silicon templates indefinitely. It takes less than a minute to make each substrate, and each substrate comes out perfectly uniform. That doesn't happen very often in nanofabrication."
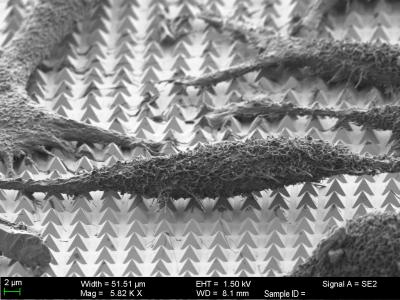
A scanning-electron microscope image of HeLa cancer cells on the substrate (Credit: Harvard SEAS)
The team cultured HeLa cancer cells directly on the pyramids and surrounded the cells with a solution containing molecular cargo. Each HeLa cancer cell sat atop about 50 pyramids, meaning the researchers could make about 50 tiny pores in each cell. The researchers could also tweak the size of the bubbles by adjusting the laser parameters, and control which side of the cell to penetrate.
According to the team, the molecules delivered into the cell were about the same size as clinically relevant cargos, including proteins and antibodies. The technology, for which patent applications have been filed, will now be tested on different cell types, including blood cells, stem cells and T cells.
"This work is really exciting because there are so many different parameters we could optimise to allow this method to work across many different cell types and cargos," said Saklayen. "It's a very versatile platform."




Project to investigate hybrid approach to titanium manufacturing
What is this a hybrid of? Superplastic forming tends to be performed slowly as otherwise the behaviour is the hot creep that typifies hot...