Typically, children are recommended to receive nine immunisation injections in their first year (five different vaccines covering 123 diseases with booster shots), and it’s fair to say that it’s rarely a pleasant experience for anyone. Massachusetts Institute of Technology medical engineering expert Prof Robert Langer — for some years the engineer most cited in primary research around the world and the 2015 winner of Queen Elizabeth Prize for Engineering — has led research that could see all those jabs combined into a single dose.
The key to the innovation is a microparticle that not only contains all the active ingredients of the immunisations, but releases them into the bloodstream in bursts at intervals that can be precisely timed by engineering how the particles are constructed, mimicking the way that the vaccines are given currently in separate doses but with only one traumatic needle.

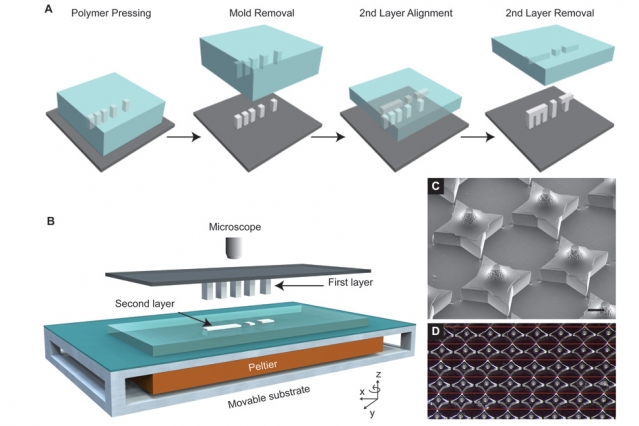
In a paper in Science, Langer and Ana Jaklenec, a researcher at the Koch Centre for Integrative Cancer Research at MIT, along with engineers Kevin McHugh and Thandh Nguyen, describe a process called stamped assembly of polymer layers (SEAL), inspired by fabrication techniques for microprocessors, that makes a structure similar in shape to a coffee cup with a lid on it.
The team first tried 3D printing to make the particles, but that proved unsuccessful. The new method uses stereolithography to create a silicon mould for both the cups and lids, sized so that 200 moulds can fit onto a standard glass slide. These moulds are used to cast cube-shaped open-sided boxes, a few hundred microns along each side, from the biocompatible, degradable polymer PGLA (polylactic-co-glycolic acid), a combination of lactic and glycolic acid units polymerised together, which is already used for medical applications. A custom-built automated device fills the cups with vaccine, then lids are lowered onto the cup and the whole assembly heated to seal it. To create the crucial multi-vaccine vehicle, several cups have to be filled with different vaccines and fused together; the molecular weight and structure of the polymer backbone of the PGLA copolymer forming each cup determines how fast the polymer degrades in the bloodstream and releases the cup’s cargo.
“Each layer is first fabricated on its own, and then they’re assembled together,” Jaklenec said. “Part of the novelty is really in how we align and seal the layers. This new method…can be used with any thermoplastic material and allows for fabrication of microstructures with complex geometries that could have broad applications, including injectable pulsatile drug delivery, pH sensors, and 3-D microfluidic devices.”
The team has tested the capsules in mice and shown that they release drugs in sharp bursts, without leakage, 9, 20 and 41 days after injection. They have also designed capsules that degrade hundreds of days after injection, although they stress that there is a challenge here in developing vaccines that will remain stable inside the capsules for that long. One particular application for this technology is in vaccines for developing countries where parents might not be able to bring small children to a remote clinic numerous times for a sequence of injections and precisely-scheduled boosters. “That might be the difference between not getting vaccinated and receiving all of your vaccines in one shot,” McHugh said.
Langer’s previous work has focused on drug delivery systems, microchip implants and tissue engineering, including the development of systems to deliver drugs to stop the development of blood vessels to tumours at precise locations within the body and drug-coated cardiovascular stents.





Red Bull makes hydrogen fuel cell play with AVL
Formula 1 is an anachronistic anomaly where its only cutting edge is in engine development. The rules prohibit any real innovation and there would be...