Sepsis occurs when the body’s immune response to infection triggers an inflammation chain reaction throughout the body, causing high heart rate, high fever, shortness of breath, and other issues. If left unchecked, it can lead to septic shock, where blood pressure falls, and organs shut down.
To diagnose sepsis, doctors traditionally rely on various diagnostic tools, including assessment of symptoms and vital signs, blood tests, and other imaging and lab tests. However, the condition can often go undetected until it is too late to effectively treat.
MIT’s new technology -which is the subject of a paper being presented at the 41st Engineering in Medicine and Biology Conference in Berlin this week (23 - 27 July 2019) works by detecting protein biomarkers that occur at very low levels at the early stages of sepsis, before other symptoms have become apparent.
Whilst these biomarkers are difficult to detect quickly using traditional assay devices, the MIT system is reportedly able to detect clinically significant levels of IL-6 - a protein produced in response to inflammation – in about 25 minutes using less than a finger prick of blood.
Traditional assays that detect protein biomarkers are bulky, expensive machines relegated to labs that require about a millilitre of blood and produce results in hours. In recent years, portable “point-of-care” systems have been developed that use microliters of blood to get similar results in about 30 minutes.
But point-of-care systems can be very expensive since most use pricey optical components to detect the biomarkers. They also capture only a small number of proteins, many of which are among the more abundant ones in blood. Any efforts to decrease the price, shrink down components, or increase protein ranges negatively impacts their sensitivity.
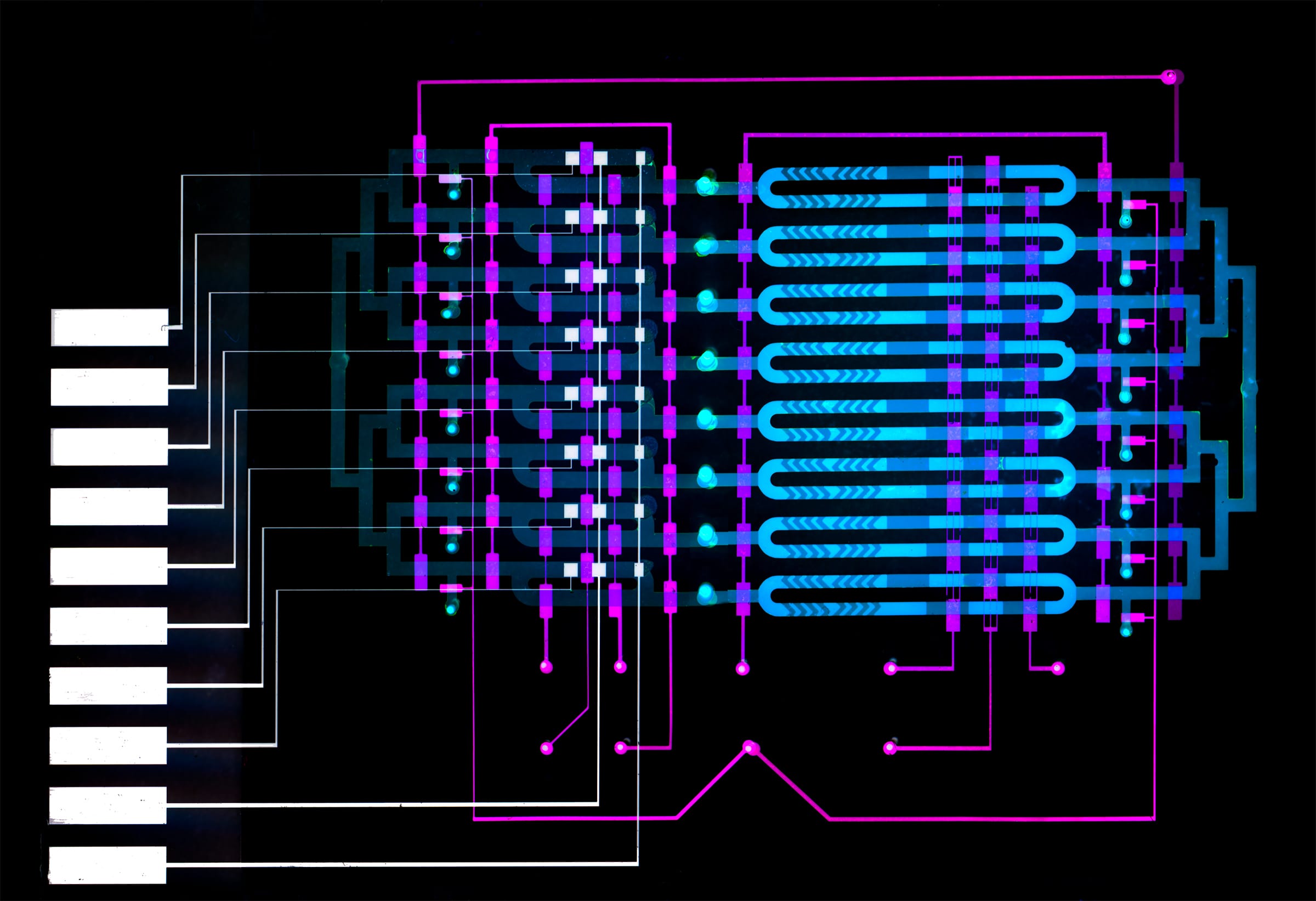
In their work, the researchers wanted to shrink components of the magnetic-bead-based assay, which is often used in labs, onto an automated microfluidics device that’s roughly several square centimetres.
In one microfluidic channel, microbeads laced with antibodies mix with a blood sample to capture the IL-6 biomarker. In another channel, only beads containing the biomarker attach to an electrode. Running voltage through the electrode produces an electrical signal for each biomarker-laced bead, which is then converted into the biomarker concentration level.
The device, which uses about 5 microlitres of blood, captures IL-6 concentrations as low as 16 picograms per millilitre, which is below the concentrations that signal sepsis, meaning the device is sensitive enough to provide clinically relevant detection.
The current design has eight separate microfluidics channels to measure as many different biomarkers or blood samples in parallel. Different antibodies and enzymes can be used in separate channels to detect different biomarkers, or different antibodies can be used in the same channel to detect several biomarkers simultaneously.
The work was funded by Analog Devices, Maxim Integrated, and the Novartis Institutes of Biomedical Research.




Red Bull makes hydrogen fuel cell play with AVL
Formula 1 is an anachronistic anomaly where its only cutting edge is in engine development. The rules prohibit any real innovation and there would be...