In contrast to energy harvested with photovoltaic cells, solar thermal energy is generally developed as a large power plant, where acres of mirrors precisely reflect sunlight onto a solar receiver. This energy is then used to heat a liquid and drive a turbine that produces electricity. However, the same storage and intermittency problems associated with traditional solar remain.
The advance - described in ChemSusChem - involves a thermochemical storage system that acts like a battery where the transfer is based on heat rather than electricity. During ‘charging’, strontium carbonate decomposes into strontium oxide and carbon dioxide, consuming the thermal energy produced by the sun. When discharging, the recombination of strontium oxide and carbon dioxide releases the stored heat.
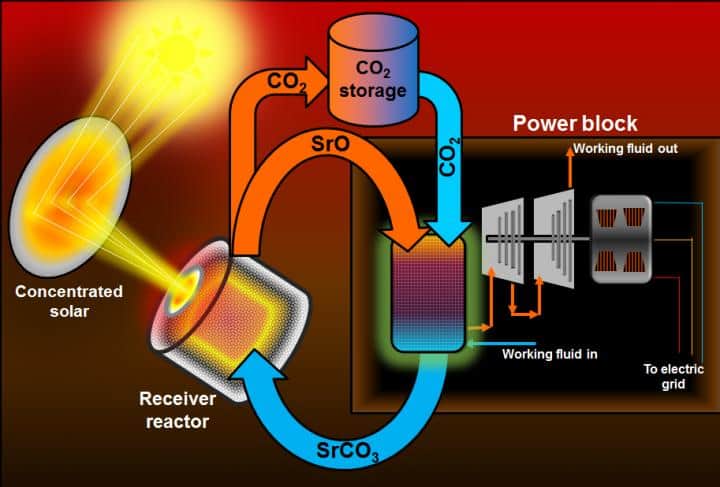
According to the researchers, these materials are non-flammable, readily available and environmentally safe. They claim that compared to existing systems, a storage device made using these materials would allow a 10-fold increase in energy density, meaning it would also be cheaper and smaller.
“With the compounds we’re studying, there’s significant potential to lower costs and increase efficiency,” said Nick AuYeung, an assistant professor of chemical engineering at OSU and one of the paper’s authors.
“In these types of systems, energy efficiency is closely related to use of the highest temperatures possible. The molten salts now being used to store solar thermal energy can only work at about 600 degrees centigrade, and also require large containers and corrosive materials. The compound we’re studying can be used at up to 1,200 degrees, and might be twice as efficient as existing systems.”
The team says that the temperatures would be so high that the system could first directly heat air to drive one turbine, and then residual heat could be used to make steam to drive another turbine.
But while there is much early promise in the work, there are also concerns over the durability of the system. In lab tests, storage capacity declined after 45 cycles due to changes in the underlying materials. The researchers are looking to significantly extend this number, as well as exploring ways that the materials could be reprocessed.




Report highlights significant impact of manufacturing on UK economy
I am not convinced that the High Value Manufacturing Centres do anything to improve the manufacturing processes - more to help produce products (using...