The new technique, developed by MIT researchers, collaborating with investigators at Beth Israel Deaconess Medical Center (BIDMC), Boston, and the IT'IS Foundation in Switzerland, sees electrodes placed onto the scalp, which has the potential to make deep brain stimulation less risky, less expensive, and more accessible.
"Traditional deep brain stimulation requires opening the skull and implanting an electrode, which can have complications,” said Ed Boyden, an associate professor of biological engineering and brain and cognitive sciences at MIT, and the senior author of the study, which appears in Cell. “Secondly, only a small number of people can do this kind of neurosurgery. "
Deep brain stimulation is used also to treat some patients with obsessive compulsive disorder, epilepsy, and depression, and are exploring the possibility of using it to treat other conditions such as autism. The new, non-invasive approach could make it easier to adapt deep brain stimulation to treat additional disorders, the researchers said.
"Current treatments are severely limited for the 127,000 people living with Parkinson’s in the UK"
"With the ability to stimulate brain structures non-invasively, we hope that we may help discover new targets for treating brain disorders," said the paper's lead author, Nir Grossman, a former Wellcome Trust-MIT postdoc working at MIT and BIDMC, who is now a research fellow at Imperial College London.
Electrodes for treating Parkinson's disease are usually placed in the subthalamic nucleus, which is a lens-shaped structure located below the thalamus located deep within the brain. For many Parkinson's patients, delivering electrical impulses in this brain region can improve symptoms, but the surgery to implant the electrodes carries risks, including brain haemorrhage and infection.
The MIT team devised a way to deliver electrical stimulation deep within the brain via electrodes placed on the scalp by taking advantage of temporal interference, which requires the generation of two high-frequency electrical currents using electrodes placed outside the brain.
According to MIT, these fields are too fast to drive neurons. However, these currents interfere with one another in such a way that where they intersect, deep in the brain, a small region of low-frequency current is generated inside neurons. This low-frequency current can be used to drive neurons' electrical activity, while the high-frequency current passes through surrounding tissue with no effect.
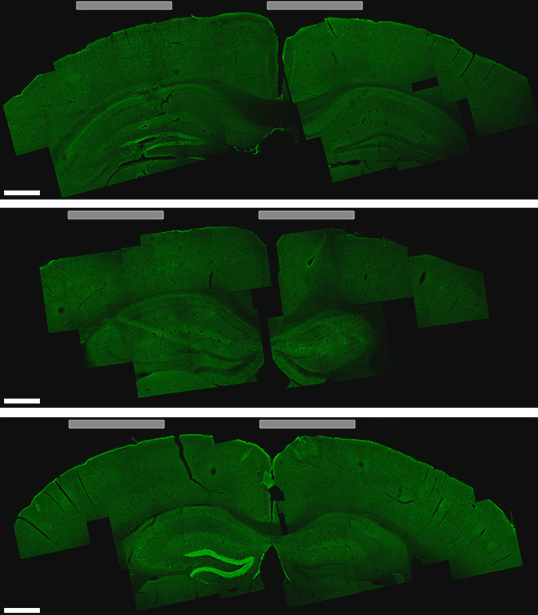
By tuning the frequency of these currents and changing the number and location of the electrodes, the researchers can control the size and location of the brain tissue that receives the low-frequency stimulation. They can target locations deep within the brain without affecting any of the surrounding brain structures. They can also steer the location of stimulation, without moving the electrodes, by altering the currents. In this way, deep targets could be stimulated, for therapeutic use and basic science research.
Commenting on the new technique, Claire Bale, head of Research Communications at Parkinson’s UK, said: "Current treatments are severely limited for the 127,000 people living with Parkinson’s in the UK.
“While the main surgical approach – deep brain stimulation – can be extremely effective, it is suitable for only a small proportion of people with the condition, partly because of its invasive nature.
"The development of less invasive techniques like this one, where electrodes are placed on the scalp rather than inside the brain, could mean that more people with Parkinson’s could benefit from this type of therapy.
"At this stage, the new technique has shown promise in mice in the lab, so there is much further research needed to develop it to the stage where it could be ready to be tested in people.”





Nanogenerator consumes CO2 to generate electricity
Whoopee, they've solved how to keep a light on but not a lot else.