Hospitals around the world have become increasingly worried about the risks of crossinfection between patients, particularly with the rise of ‘super bugs’ resistant to antibiotics.
Alarmingly, research indicates that one in 10 patients will acquire an infection during their hospital stay.
A first-of-its-kind study at Leeds University, by the Pathogen Control Engineering (PCE) research group in the School of Civil Engineering, has found that making simple changes to the design of hospital wards and ventilation systems could be a critical factor in controlling outbreaks of serious airborne infections, such as SARS, influenza and tuberculosis, as well as some well-known hospital pathogens like acinetobacter and MRSA.
The study’s findings will inform the UK’s new guidelines for the ventilation of healthcare facilities.
'There is evidence that 10 to 20% of infections are spread through the air, but until now, their role in the infection chain has been largely overlooked, as doctors tend to emphasise the importance of washing hands and avoiding physical contact,' said principal investigator Dr Andrew Sleigh.
'Although diseases such as tuberculosis are widely accepted as being airborne, others may also be spread this way. Numerous bacteria-carrying particles - such as tiny flakes of human skin - can be widely dispersed into the air within hospital wards through routine activities, and potentially contribute to the risk of infection for patients,' he added.
To investigate this further, PhD research students Katherine Roberts and Abigail Hathway used a laser counter to measure the number and size of particles present in the air of a respiratory ward at St James’ University Hospital, taking air samples every 30 minutes over a working day. They also sampled for micro-organisms in the air using a bioaerosol sampler, and kept a log of activities within the ward.
They were able to show that human activities such as ward rounds, changing the beds, drawing the curtains, and patients using nebulisers were linked to peaks in the number of airborne particles in the ward.
To understand the implications of such findings, the PCE research group has brought together engineers, microbiologists, mathematicians and medical experts to examine the problem from several different perspectives.
This is apparent in other aspects of the study, where computer modelling has yielded considerable insight into the risks of airborne infection.
Civil engineering lecturer Dr Catherine Noakes used computational fluid dynamics to model a tuberculosis ward in Peru. This allowed her to predict how airborne particles would travel from one patient’s bed to another, spreading pathogens across the room.
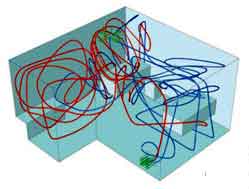
These diagrams show the flowpaths of contaminated air coming from two patients (red and blue lines) in a hospital ward, before (above) and after (below) modifying the ventilation inlets/outlets, and placing a partition between the beds. The air ventilation rate remained constant, yet transfer of infectious material between the two beds was reduced by over 75%.
The model showed that moving the ventilation inlets and outlets to different positions could reduce the risk of airborne transmission by more than 75%, without having to increase the air supply into the ward, which would consume more electricity.
Dr Noakes also calculated the risks of an airborne disease outbreak in a hospital environment, accounting for factors such as temperature and humidity. This showed that in some cases, less than a 10% increase in the ventilation rate may be sufficient to prevent a major outbreak.
The research was funded by the Department of Health’s NHS Estates and the Engineering and Physical Sciences Research Council.
To read about other projects led by the Pathogen Control Engineering research group, click here.




Swiss geoengineering start-up targets methane removal
No mention whatsoever about the effect of increased methane levels/iron chloride in the ocean on the pH and chemical properties of the ocean - are we...