Published in the journal ACS Sensors, the new study explores using a ‘smart bandage’ with electroanalytical methods of monitoring certain biomarkers during the healing process, all without the need for bandage removal.
To monitor healing in chronic wounds, such as diabetic foot ulcers or pressure ulcers, medical professionals usually need to remove the bandages from a wound. This damages recovering tissue, can be painful for patients, and requires hospital visits to avoid further infections. If a wound requires further inspection, other methods such as biopsies, surface swabs or testing for pathogens can be invasive, costly and sometimes ineffective.
Leader of the Russia-US team, Prof. Keith Stevenson, said that their methods could be particularly promising for clinical application due to their simplicity, sensitivity and durability.
“Earlier stages of our research involved characterising the sensor performance and demonstrating the sensitive and selective multianalyte detection in complex biofluid simulants that closely mimic real biological environments,” he explained.
Sensors printed directly onto human skin without heat
Transparent fibre sensors 'smell, hear and touch'
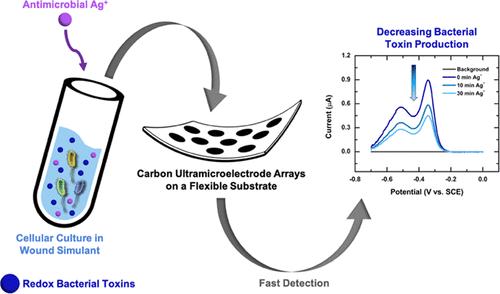
According to researchers, an early prototype of an electroanalytical wound sensor was built based on carbon ultra- microelectrode arrays (CUAs) on flexible substrates. In previous studies, this sensor had been placed on quartz substrates, but to ensure flexibility, the authors developed a method of putting the arrays on a polyethylene terephthalate (PET) substrate.
The team said they used a simulated wound environment to test the sensitivity of their sensor to three critical biomarkers: pyocyanin, produced by Pseudomonas aeruginosa, a bacterium typically colonising chronic wounds; nitric oxide (NO) secreted in response to bacterial infections by cells of the immune system; and uric acid, a metabolite which strongly correlates with the severity of a wound. These compounds are electroactive, meaning they respond to electrical activity and can be detected by an electroanalytical sensor.
Results showed that the sensor's limits of detection and linear dynamic ranges, which represent the ranges of concentrations where a sensor produces meaningful quantitative results, were within the biologically relevant concentrations, meaning a device based on these sensors could be used for wound monitoring in a clinical setting.
The researchers also tested their sensor in cell cultures, where it successfully detected pyocyanin from P. aeruginosa and NO from macrophages (immune cells that destroy bacteria and other 'invaders'). Finally, they confirmed the sensor was also able to detect the influence of Ag+ silver ions, a known antimicrobial agent, that suppressed pyocyanin production by the bacteria.
Prof. Stevenson said that the next steps for the team will be to utilise the technology in real-time monitoring of wound treatment effectiveness on human subjects in clinical settings.





Red Bull makes hydrogen fuel cell play with AVL
Formula 1 is an anachronistic anomaly where its only cutting edge is in engine development. The rules prohibit any real innovation and there would be...