Ultrasound could be used to image cells and molecules deep inside the human body thanks to developments in protein engineering at Caltech in the US.
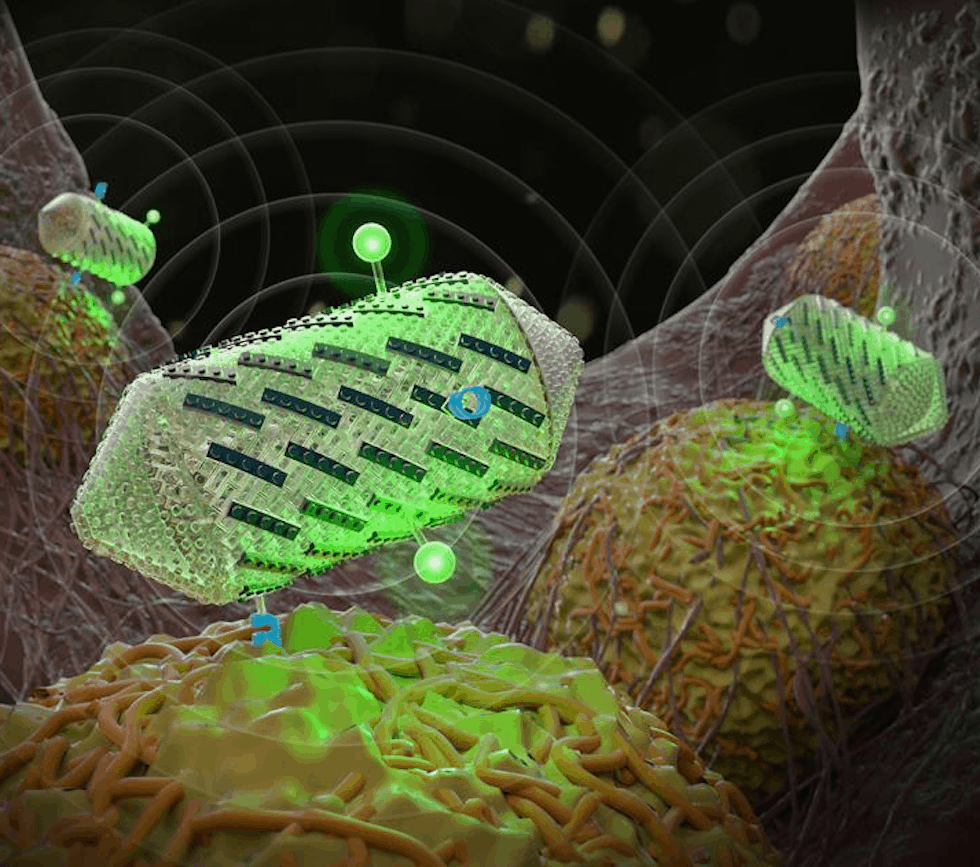
The researchers are said to have engineered protein-shelled nanostructures called gas vesicles - which reflect sound waves - to exhibit new properties useful for ultrasound technologies.
The modified gas vesicles were shown to give off more distinct signals, making them easier to image; target specific cell types; and help create colour ultrasound images. In the future, they could be administered to a patient to visualize tissues of interest.
"It's somewhat like engineering with molecular Legos," said Mikhail Shapiro, an assistant professor of chemical engineering and senior author of a new paper about the research published in ACS Nano. "We can swap different protein 'pieces' on the surface of gas vesicles to alter their targeting properties and to visualise multiple molecules in different colours."
"Today, ultrasound is mostly anatomical," said Anupama Lakshmanan, a graduate student in Shapiro's lab and lead author of the study. "We want to bring it down to the molecular and cellular level."
Gas vesicles are naturally occurring in water-dwelling single-celled organisms, such as Anabaena flos-aquae, a species of cyanobacteria that forms filamentous clumps of multicell chains. The gas vesicles help the organisms control how much they float and their exposure to sunlight at the water's surface. From a previous study, Shapiro realised that the vesicles would readily reflect sound waves during ultrasound imaging, and demonstrated this using mice.
In the current research, Shapiro and his team set out to give gas vesicles new properties by engineering gas vesicle protein C, or GvpC, a protein naturally found on the surface of vesicles that prevents them from collapsing. The protein can be engineered to have different sizes, with longer versions of the protein producing stronger and stiffer nanostructures.
"The proteins are like the framing rods of an airplane fuselage. You use them to determine the mechanics of the structure," Shapiro said in a statement.
In one experiment, the scientists removed the strengthening protein from gas vesicles and then administered the engineered vesicles to mice and performed ultrasound imaging. Compared to normal vesicles, the modified vesicles vibrated more in response to sound waves, and resonated with harmonic frequencies. According to Caltech, harmonics are not readily created in natural tissues, making the vesicles stand out in ultrasound images.
In another set of experiments, the researchers demonstrated how the gas vesicles could be made to target certain tissues in the body. They genetically engineered the vesicles to display various cellular targets, such as an amino acid sequence that recognises proteins called integrins that are overproduced in tumour cells.
The team is also said to have shown how multicolour ultrasound images might be created. Conventional ultrasound images appear black and white. Shapiro's group created an approach for imaging three different types of gas vesicles as separate "colours" based on their differential ability to resist collapse under pressure. The vesicles themselves do not appear in different colours, but they can be assigned colours based on their different properties.
To demonstrate this, the team made three different versions of the vesicles with varying strengths of the GvpC protein. They then increased the ultrasound pressures, causing the variant populations to successively collapse. As each population collapsed, the overall ultrasound signal decreased in proportion to the amount of that variant in the sample, and this signal change was then mapped to a specific colour. In the future, if each variant population targeted a specific cell type, researchers would be able to visualise the cells in multiple colours.
"You might be able to see tumour cells versus the immune cells attacking the tumour, and thus monitor the progress of a medical treatment," said Shapiro.




Red Bull makes hydrogen fuel cell play with AVL
Formula 1 is an anachronistic anomaly where its only cutting edge is in engine development. The rules prohibit any real innovation and there would be...