Methanol fuel is used to power the electronics while carbon dioxide (CO2), produced as reaction by-product, pumps the liquid analytes around the channels.
Microfluidic technologies have been touted for some time, and while small devices have been demonstrated they typically rely on large and expensive external equipment such as compressors and various electronic components.
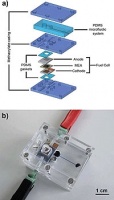
This essentially renders them unsuitable for applications such as point-of-care diagnostics and field work in developing nations.
Around six years ago, a team of Spanish researchers from the National Centre for Microelectronics (CNM) in Barcelona came across the lab-on-a-chip problem.
‘We attended two or three microTAS [Micro Total Analysis Systems] conferences and saw that there were many platforms and a lot of technologies but not many commercial products,’ Dr Neus Sabaté of the CNM told The Engineer.
‘The two main reasons seemed to be the lack of autonomy of these devices and the lack of consensus of which was the best technology to put everything together.’
At this stage, the researchers began investigating micro fuel cells, at a time when there was talk of them being used in laptops and mobile phones.
‘It’s clear now that micro fuel cell research has lost the battle against batteries because lithium-ion batteries are getting better and better. But we think we’ve found a niche for micro fuel cells with lab-on-a-chip devices,’ Sabaté said.
In the current prototype device, the micro fuel cell is composed of two silicon current collectors and a commercial membrane electrode assembly (MEA). The collectors are fabricated with deep reactive ion etching (DRIE) to allow the reactants to distribute by diffusion.
When the micro fuel cell starts to deliver electrical power to a certain load, CO2 gas is created at the anode side as a result of the oxidation of methanol molecules. The accumulation of CO2 in the inlet chamber causes a pressure increase that forces the liquid in the sample chamber to displace towards the analysis chamber.
‘We’re taking advantage of these by-products, which are generally a nuisance for these kind of devices, and turning it into mechanical power,’ Sabaté said.
The researchers hope to further develop their current prototype device, but Sabaté believes there may be even greater potential for micro fuel cells that can take sugar water and even variants powered by the very same fluids they are analysing.
In addition, they are working on a device where the entire platform — both the fuel cell and the chip — are made from the same biodegradable polymer, making a much more flexible, robust device.





Glasgow trial explores AR cues for autonomous road safety
They've ploughed into a few vulnerable road users in the past. Making that less likely will make it spectacularly easy to stop the traffic for...