The viscosity of different parts of cancer cells increases dramatically when they are blasted with light-activated cancer drugs, according to new images that provide fundamental insights into how cancer cells die.
The images, taken by researchers from Imperial College London, reveal the physical changes that occur inside cancer cells while they are dying as a result of Photodynamic Therapy (PDT). This cancer treatment uses light to activate a drug that creates a short-lived toxic type of oxygen, called singlet oxygen, which kills cancerous cells.
The research team behind the study says that revealing what happens to viscosity within a dying cancer cell is important because it helps give a better understanding of how cells function and which factors are important for controlling reactions inside cells. Ultimately, this could help scientists design more efficient drugs for Photodynamic Therapy and other treatments.
The research is also of wider significance because these are the first ever real-time maps showing viscosity changing over a period of time inside a cell during a biologically important process such as cell death.
Previous studies have shown that the viscosity of human cells and organs also changes in patients with diseases such as diabetes and atherosclerosis, said Dr Marina Kuimova from Imperial College London's Department of Chemistry, who carried out the research.
'We're still not quite sure exactly what the relationship is between increased stickiness inside cells and disease, but we expect that the two are related,' added Kuimova.
'Knowing more about these changes, and being able to map them when they occur in all kinds of different scenarios, from dying cancer cells, to diseased blood cells, could help us to better understand how some diseases and their treatments affect cell and organ function.'
Dr Kuimova and her colleagues were able to track viscosity as it changed inside live cancer cells thanks to a newly developed Photodynamic Therapy drug, with unusual fluorescent properties. The drug, which is made of a molecule with a spinning component like a rotor, emits different wavelengths of light depending on the viscosity of its surroundings.
The changing wavelengths of light emitted during experiments, and captured over a period of 10 minutes, showed that once the PDT drug was activated, the level of viscosity inside the cell increased dramatically. The researchers suggest that this increasing viscosity is caused by the toxic oxygen molecules released into the cell.
They think that increased levels of viscosity might even contribute directly to the cancer cell's further deterioration by slowing down vital communication and transport processes inside the cell.
Dr Stanley Botchway from the Science and Technology Facilities Council, which worked in collaboration with Imperial College London on the research, said: 'The huge viscosity we measured was surprising and it certainly gives a new insight into the change in cellular environment during cell death.'
However, the researchers noted that as viscosity in the cancerous cell increases, the toxic oxygen molecule's mission to kill the cell is slowed down too.
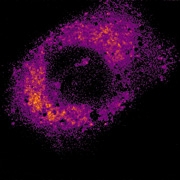
Dr Kuimova explained: 'It looks like while the increasing viscosity contributes to the cell's demise, these new "sticky" cell conditions can slow the drug down, so it’s not as straightforward a relationship as it might first appear.
'More work is needed to better understand the complex interplay between viscosity and cell death. We hope to use our imaging technique to track changes in viscosity in other kinds of cells as they occur in real time, to unlock some of the secrets of what goes on inside cells when they're functioning, malfunctioning or dying.'
The research was led by Imperial College London in collaboration with the Science and Technology Facilities Council's Rutherford Appleton Laboratory (RAL), Oxford University, King's College London, and the University of Aarhus in Denmark.
The work was funded by the Engineering and Physical Sciences Research Council, with support from the Science and Technology Facilities Council, and the Danish Foundation for Basic Research.




Project to investigate hybrid approach to titanium manufacturing
Sadly they will not be ordering any more presses from Wilkins & Mitchell http://www.historywebsite.co.uk/articles/Darlaston/WM.htm