A little GEM
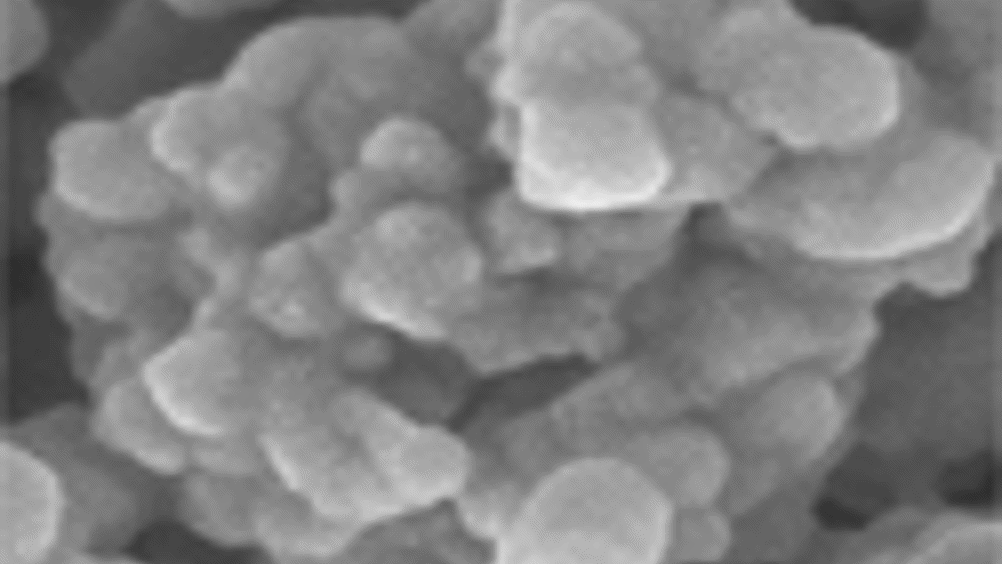
BioMimetic Therapeutics has recently initiated three clinical trials to evaluate GEM OS1 Bone Graft for use in foot and ankle fusion procedures and the treatment of unstable distal radius fractures.
GEM OS1 is a bioactive drug-device combination product that combines a tissue growth factor, recombinant human Platelet-Derived Growth Factor (rhPDGF), one of the principal wound healing stimulators in the body, with a synthetic bone matrix, Beta-tricalcium phosphate (Beta-TCP).
BioMimetic is leveraging the same combination drug-device technology for orthopaedic applications as it utilised in its FDA approved product, GEM 21S Growth-factor Enhanced Matrix for the treatment of periodontally related defects and gingival recession.
‘As a foot and ankle surgeon, I see a significant number of lower extremity fusion procedures that either do not heal or heal slowly, often leading to prolonged recovery times, increased patient morbidity, and greater cost to the patient and health care system,’ said lead US investigator, Dr. Christopher W. DiGiovanni, chief of foot and ankle in the department of orthopaedic surgery at Rhode Island Hospital and associate professor at Brown Medical School in Providence, RI.
Register now to continue reading
Thanks for visiting The Engineer. You’ve now reached your monthly limit of news stories. Register for free to unlock unlimited access to all of our news coverage, as well as premium content including opinion, in-depth features and special reports.
Benefits of registering
-
In-depth insights and coverage of key emerging trends
-
Unrestricted access to special reports throughout the year
-
Daily technology news delivered straight to your inbox










Fusion inches closer as ITER completes magnet system
I believe the purpose of ITER isn't to make usable power, it is a research project which will be used to design the first generation of actual...