In 1780, Italian scientists Lucia and Luigi Galvani performed one of the earliest and most famous experiments in bioelectricity, applying an electrical charge to a dead frog that caused its leg muscles to twitch. Nearly two and a half centuries later, the field of bioelectronics has evolved, though many applications have remained confined to the laboratory. That is about to change.

To this point, internal electrical stimulation has been somewhat limited, with pacemakers for the heart and deep brain stimulation (DBS) for Parkinson’s and epilepsy probably the best-known examples. Now a new wave of biomedical companies is seeking to expand the field, hacking the body’s codes to treat everything from arthritis and diabetes to cancer, cardiovascular disease and asthma.
At the forefront of this wave is Galvani Bioelectronics – its name a nod to the Italian couple who pioneered those first amphibian experiments. A joint venture between pharma giant GSK and Verily (formerly Google Life Sciences), the company has both the pedigree and the pockets to catapult bioelectronics forward, carving out an entirely new therapeutic segment in healthcare.
“There are different ways in which you can plug into biology or physiology and have a treatment effect,” Kristoffer Famm, President of Galvani Bioelectronics, told The Engineer. “If you look at sort of textbook biology, it’s very clear that there is molecular control of a number of things in our cells and organs - and then there is electrical control.
“In the pharmaceutical and the broader treatment world, we’ve been very focused on the molecular control and how you can piggyback on that... whereas the electrical has played second fiddle.”
Prior to leading Galvani, Famm headed up GSK’s bioelectronics R&D department, which he helped found back in 2013. Though his background is in chemical engineering and molecular biology, for almost a decade, he has been investigating how bioelectronics could be used to replace or enhance traditional pharmacological treatments and, perhaps more importantly, tackle some of the medical mysteries that drugs have so far been unable to solve. In GSK’s early work, Famm and his colleagues looked at using electrical signals to stimulate or block aspects of the nervous system in animal models.
“It became very clear that we could have a very meaningful effect on disease state and that ranged from things like rheumatoid arthritis...to diabetes to asthma and smokers cough and so on,” he said.
Every human organ in the central body cavity has nerve networks and, according to Famm, these can essentially be hacked to control the body’s response to an enormous range of conditions. But to do so requires a refined new style of implant, one specifically designed to manipulate these nerves, that is easily insertable, easily charged by the user and programmable by clinicians.

“We need to build a new class of active implantable electronic devices that are geared towards addressing these particular nerves...different designs, different surgical implantation procedures and so on,” said Famm.
Enter Google. While GSK was investigating the potential for electrical signals as medical conduits, Alphabet-offshoot Verily – formerly part of Google’s secretive X Development division - was exploring next-gen, low-power electronics - the exact type of hardware required to deliver bioelectronic therapies.
“GSK had the therapeutic application space and the knowhow to bring this through to actual treatments to patients,” said Famm. “Verily had the technology building blocks and a lot of engineering, and the vision of bringing forward a new class of treatments.
“So we basically combined forces, both contributed capital, both contributed intellectual property, technology building blocks, biology building blocks, if you will.”
Forging this new path in a largely unmapped area of medical science takes no small amount of time and money, so it helps when your parent companies are GSK and Google. Over the past five years, that backing has helped push Galvani to the cusp of something momentous, with the first clinical trials getting underway in January 2022.
Targeting rheumatoid arthritis (RA), the trial will see patients implanted with Galvani’s neuromodulation system, sending pulses to the splenic nerve. The nerve in turn sends signals to the spleen itself, signals that should switch off the inflammation state of splenic immune cells, theoretically reducing inflammation in the joints around the body and the pain that comes with it. If the trials are successful, a whole range of other diseases, from MS and Crohn’s to hypertension and diabetes, could be targeted next.

“We’ve come to this really exciting point now,” said Famm. “We’re testing it in first diseases. The platform potential is still there and this is really important with testing it in rheumatoid arthritis. But the vision is still very much that this can apply to many different - what we call nerve targets - many different areas that can be beneficial in disease.”
In this respect, bioelectronics offers a paradigm shift from pharmacology, with the underlying technology almost certain to be adaptable for different diseases. Drug discovery generally produces highly targeted pharmaceuticals that treat one specific condition. When scientists are searching for the next miracle molecule, they begin with a blank slate, previous discoveries counting for little. Bioelectronics could turn that model on its head.
“What I was used to as a protein scientist is that in molecular drug discovery you have very little modularity. You sort of start from scratch with your next drug,” said Famm. “With this device system, you actually have a platform. You know, 80 per cent of what we build applies to the next therapy and that can be incredibly powerful, subject of course to us being successful in the first use cases. And that’s why the clinical trials that now are under way are such bellwethers.”
Galvani chose RA for its first trial for several reasons. R&D in animal models had shown robust clinical efficacy, making it a good bet for translation in humans. The splenic nerve is also an ideal target, as it can be reached via laparoscopic surgery. According to Famm, Galvani’s is the first implantable modulation device to be delivered using the minimally invasive technique.
Pulse dosage is set by a rheumatologist using a smart device and Bluetooth. Though it cannot be altered by the patient, users do have the option of switching off stimulation completely at any time. Wireless charging, facilitated by an external power device worn in a belt over the implant, is required for roughly one hour per week, although this will vary for each patient according to dosage.
“The couple of patients that are implanted now, they are literally the first patients ever to have a new modulation device stimulated directly on their splenic nerve to their spleen,” said Famm. “Hats off to those patients, they are the pioneers at the moment and it will be critical to see how the clinical studies with the first few handfuls of patients play out.”
Another major disease that Famm mentions is Type 1 diabetes, where he says Galvani’s technology has already had “transformational effects” in animal models, essentially delivering a “functional cure”.
“If we can even get close to that in man, I mean it would be game changing, right?”
As well as being a radically different way of treating disease, bioelectronics could transform what’s known as dosing compliance. Even with the best intentions in the world, we are all capable of forgetting to take a pill. Having a digitally programmed, automated delivery method of treatment promises substantial therapeutic upsides.
For certain conditions, dosing compliance can have profound effects. In Manchester, startup QV Bioelectronics is developing an electrotherapy implant to slow the growth of glioblastoma, the most lethal form of brain cancer. One of the key benefits of QV’s GRACE implant will be the continuous nature of the electrical stimulation it can deliver to the desired area of the brain, working around the clock to impede tumour regrowth.
“This is a terminal disease and has amongst the worst outcomes of any type of cancer,” explained Dr Chris Bullock, co-founder and CEO at QV Bioelectronics. “In general in the UK and around the world, the standard treatment at the moment consists of surgery to remove as much of the tumour as possible.”
Even with intensive chemo- and radiotherapy, average survival time for glioblastoma is just 14 months - a brutally brief time in the context of a life and a figure that modern medical science has had little success in extending. One treatment that has been effective in prolonging life is electric field therapy, where specific frequencies of electrical stimulation are used to disrupt the cancer’s progress after surgery, hampering cell division.
“So the five-year survival rate - the percentage of patients still alive five years after their diagnosis with the standard treatment - is about 3.5 per cent. When you add the electric field therapy to that standard treatment, it’s 13 per cent, so that’s obviously a huge step forward.”
Currently, electric field therapy involves bulky external equipment that reduces mobility and adversely impacts quality of life during treatment, and which inevitably has a negative effect on dosing compliance.
“They’re wearing this cumbersome conspicuous thing all day every day, and you know that can affect the willingness of patients to undergo the treatment,” said Bullock. “The current system is prescribed for 16 hours a day, but actually you see that the longer each day the patients undergo therapy, the better they do.
QV’s GRACE device - implanted during the same surgery to remove glioblastoma tissue - will deliver discreet, localised electric field therapy around the clock, hopefully extending life for patients while also improving the quality of that life.
“You can’t see anything from the outside,” said Bullock. “There’s no sensation from within the brain... you can’t feel anything that’s happening inside your brain. So we believe it will be a pain free, side effect-free therapy.”
Speaking to The Engineer, Dr Bullock was keen to point out that although QV’s technology has the potential to be groundbreaking, it is still at the experimental stage and unlikely to be available for several years. It is also, at the end of the day, a life-extending therapy for the terminally ill, rather than any sort of miracle cure. QV does not want to give false hope to families and patients who may currently be dealing with a glioblastoma diagnosis and facing up to the difficult path that lies ahead.
“It’s still a number of years away from being ready for human usage,” he said. “We’ve seen with other companies some quite heart-breaking situations with, you know, understandably desperate people getting in touch with companies for things that just aren’t going to be available to them.”

The road to clinical use is a long and challenging one. Medical devices of all stripes have stringent regulatory requirements, with those that operate within the body understandably having some of the most demanding. But implanted devices have been in use for several decades, so there is regulatory precedent that can help pave the way for this novel crop of bioelectronic devices, even when dealing with the most complex organs like the brain.
“I think the greatest challenge,” said Bullock, “is that we don’t really understand how the human body works, and particularly the brain. As engineers, you know, you’re trying to generate targeted effects, but when you don’t understand the full complexity of the system, that is a real challenge.”
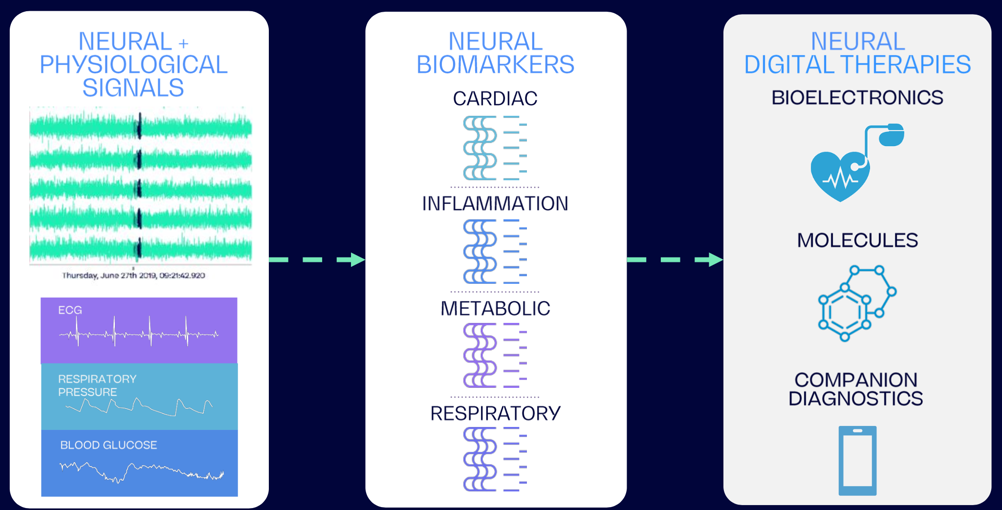
Founded in 2015, Cambridge-based BIOS Health is hoping to address that very challenge. Using neural interfaces and artificial intelligence, the company is aiming to codify the body’s biomarkers and neural messaging and develop a type of digital language that can be used both to understand and to better treat diseases.
“We really wanted to bring techniques like AI and machine learning to what felt like a fundamentally digital data problem in the body,” BIOS Health co-founder and chief scientific officer, Oliver Armitage, told The Engineer. “Something that is nice about neural interfaces is that they interact on an almost purely, digital basis with a piece of biology - the biology information is electrical and you kind of receive it digitally.”
Whereas Galvani and QV Bioelectronics are predominantly hardware-focused, BIOS is looking at the underlying neural software that runs the body, decoding those digital signals to improve health outcomes. It’s a complementary technology that could help push the whole field of bioelectronics forward, for example by helping the likes of Galvani expand into different disease areas. But tapping into and understanding real-time neural signals is no simple task.
“It’s sort of like a real-time analogue to the genome,” said Armitage. “The genome is this hard drive of data storage that you can interact with... the neural data is a similar kind of data, it’s just flowing in real time.”
BIOS’s core technology, a neural data analytics software platform, runs on a combination of local and cloud software. It essentially decodes the raw neural data, providing straightforward, actionable insights for clinicians.
According to Armitage, many of today’s existing neuromodulation devices require clinicians to moonlight as amateur electronic engineers of sorts, tweaking manual settings for things like frequency, current and pulse duration to achieve a desired medical effect. BIOS intends to eliminate the need for expert domain knowledge in electronics, allowing medical professionals to focus on patient effects rather than the intricacies of electrical signals.
“Ultimately, bioelectronic devices have become more complicated than a doctor can understand. That’s not the doctor’s fault, that’s the engineer’s fault,” he said. “I’ve seen devices where clinicians have to write the memory register where the stimulation waveform is saved. That’s a huge level of technical burden on the clinician.
“If you want the patient and the doctor to have the best experience, you should help them.”
Enhanced understanding of the body’s underlying code will not only make life easier for doctors, it will also lead to more personalised bioelectronic therapies.
“There are just different sets of stimulations that are going to result in the best effect for you, for me or anyone else,” said Armitage. “So if you want to get the best efficacy and best outcomes, you need to personalise all those settings…we basically make those personalised settings available and take that complexity away and give the doctor tools that they can actually use.”
Though BIOS’s platform is designed to help treat an array of conditions, its immediate focus is on cardiovascular disease. The company recently secured CA$1.8 million for a research partnership in Canada with Mila, McGill University and the Université de Montréal, developing an AI-controlled closed-loop neuromodulation system for chronic cardiac conditions.
“We’re working through a list...cardiac, respiratory, inflammatory, metabolic and neurological,” said Armitage. “But for us to be able to make a stimulation and affect the cardiac system and not anything else, you have to understand all those other things."
A new era of electrical medicine beckons. Quite the leap from Galvani’s frog.






Hard hat mounted air curtain adds layer of protection
Something similar was used by miners decades ago!