
With chronic kidney disease thought to affect as much as ten per cent of the global population, haemodialysis - the process of filtering the blood outside of the body - is a critical life-saving treatment for an estimated three million people globally.
And as populations around the world become older, and the prevalence of type 2 diabetes increases, the number of patients requiring this life-saving treatment is expected to grow in the years ahead.
Currently, dialysis is not without its own problems. Indeed, preparing a patient for the process involves a significant surgical intervention that carries its own set of risks for the patient and incurs significant costs.
But London-based startup Pathfinder Medical, is hoping to change all of this, by bringing the benefits of minimally invasive surgery to a field of intervention that has changed little in decades.
Based on an invention made by founder and CEO Sorin Popa, the firm’s technology uses a precision-guided catheter device to enable surgeons to connect blood vessels together without opening up the patient.
Talking to The Engineer earlier this autumn (October 2020) Popa – the recent recipient of the Royal Academy of Engineering’s special award for young engineers, the Sir George Macfarlane Medal - said that the technology, known ePATH could replace more than 600,000 surgical procedures every year, and lead to vastly improved outcomes for patients.
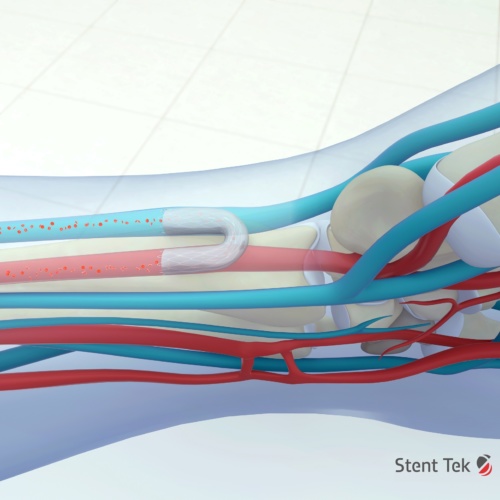
Currently, in order to get a patient ready for dialysis it’s necessary to form a special kind of blood vessel in the arm known as an arteriovenous fistula (AV fistula), which is created by connecting an artery and a vein. This dramatically increases the blood flow in the vein making it easier to transfer blood into the dialysis machine.
This fistula is typically created using an invasive surgical method that has been the norm since it was pioneered in the 1960s. However, the clinical outcomes from this procedure are very poor. Indeed, around a quarter of the connections fail immediately, and even when they are successful, patients require on average 3.4 procedures per year to keep the surgical fistula running.
They also take a long time to heal, meaning that patients sometimes have to wait for a couple of months before dialysis can begin. “It’s the weakest link in dialysis treatment,” said Popa, “fundamentally there are inherent problems with a surgical procedure like that where you have to dissect vessels out and that shows up in the outcomes.”
We’ve learned that getting from a prototype that works, to a medical device that’s ready for the clinic is an enormous step
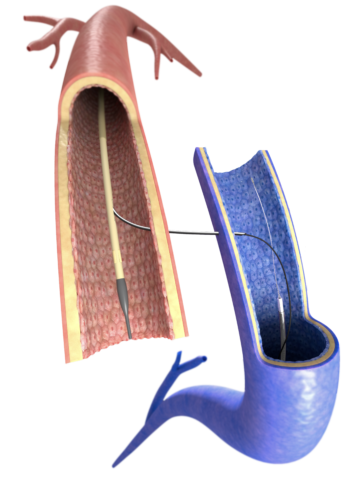
Pathfinder’s technology replaces the need for surgery with a so called endoAVF technique (endovascular arteriovenous fistula) that uses two electronically guided catheters which are navigated through a patient’s blood vessels, aligned electronically, and used to precisely pass a needle between vessels that are centimetres apart.
Popa explained that during use, a clinician will monitor the real time progress of the catheters using a system that he likens to a GPS for navigating the blood vessels.
One catheter contains a needle in a retracted position, whilst the second device acts as a target. When the needle draws level with the target site, the clinician receives an alert to deploy the needle, which can then cross accurately from one vessel to another. Once the needle has traversed the gap between the two vessels, a wire is passed between them, and a stent can be passed over the wire to form the connection between the vessels.
“The end result is same as from a surgical fistula,” said Popa. “But instead of opening up the arm, it’s all done with a small needle that’s less than 1mm in diameter, so it’s very low impact.”
Whist the procedure is expected to be much quicker than the current surgical technique, its key benefit - based on the performance of competing Endo AVF systems - is expected to be vastly improved clinical outcomes. “Existing technology suggests that minimally invasive fistulas last much longer and require fewer interventions to maintain the function,” said Popa.
Another benefit is that, compared to the complexities of open surgery, it’s relatively easy to use, something which potentially opens up the treatment to a wider range of patients. “The surgical procedure to create a fistula takes a lot of training and you’re dealing with small 3mm vessels that need to be sutured which requires quite a skilled surgeon to do,” said Popa. “This crossing guidance requires skilled intervention, but this crossing that would otherwise be impossible to do becomes very easy.”
Popa’s team isn’t the first to consider the potential of minimally invasive surgery in this field. Indeed, tools developed by TVA Medical and Avenue Medical (both of which have since been acquired by larger companies - BD and Medtronic) have already gone onto the market.
However, according to Popa these competing technologies work in a slightly different way and are limited to working in the upper forearm where vessels are very close together. The preferred first line option for fistulas is nearer the wrist, where the vessels are further apart, which is where Pathfinder Medical’s technology comes in.
The system began life when - as part of the first cohort of Imperial Collage London’s medical device design entrepreneurship programme – Popa was first introduced to this clinical need of navigating alignment and crossing vessels in a minimally invasive way. “My invention was based on my electrical engineering background and was using electric fields to align,” he said, “and that’s what really enabled getting this very precise and low cost way of guiding that needle between vessels.”
He then made a crude prototype to demonstrate the concept and rapidly concluded that it had a huge amount of promise. “It’s very low power, it’s very safe. There’s no risk to the patients. It’s very easy in principle to manufacture. It’s very precise. There’s quite a high signal to noise ratio and It’s not impacted by the body or vessels that may be diseased.”
However, coming up with the idea was the easy part. “We’ve learned that getting from a prototype that works, to a medical device that’s ready for the clinic is an enormous step. There’s a huge amount of testing and also the way you approach the engineering side is quite different. The materials you can use and things that are easy to do in a lab and to prototype become very different to do reliably with the right clinical grade processes.”
Producing the electronics was, he said, particularly challenging. “It’s very easy to make a prototype that works. But to get electrodes printed onto a catheter was actually quite a challenge and it took us a long time to find the right people and the right process to do that reliably.”
Finally, six years after founding the company, the journey is entering an exciting new phase, and with regulatory approval now in place in the form of a CE mark, Popa and the team are moving ever closer to their goal of revolutionising dialysis treatment.
Before the COVID-19 lockdown, the team was already involved in its first clinical study – which was exploring the use of the technology to bypass blockages in the legs, and the group hopes to recommence this work shortly.
More surgical innovations from The Engineer
How Versius could bring keyhole surgery to the masses
However, whilst there are a number of potential applications for the system, the key aim for the moment, said Popa, is to focus on the dialysis applications and to put in place a clinical study that will provide the data required to get the technology on the market. “We know that it works and we know that it’s safe,” he said. “We’ve done pre-clinical work so the next step is to try a large study and show the clinical outcomes we expect will actually occur.”
If all goes to plan, he hopes that within two years the company could have all the data it needs to get the technique on the market, and at that point he anticipates either becoming acquired by or partnering with a larger med-tech firm which will be will be able scale up and fully commercialise the technology. “Our job is to develop the technology, to show that it works and that the clinical outcomes are there, and then commercialisation is best left to those who have the commercial strength to get this out globally and really impact patients around the world.”
In the meantime, he’s enjoying the thrill of working in one of engineering’s most rewarding and worthwhile sectors. “What I’ve always liked about medtech is that I see it is as a win, win, win area,” he said. “You can’t get technology adopted unless it’s much better for the patients and you’re reducing costs for the healthcare system as a whole. Only then can you have a commercial success. I like that aspect of it. It’s quite fulfilling and it doesn’t feel like we’re just trying to sell things.”











Water Sector Talent Exodus Could Cripple The Sector
Maybe if things are essential for the running of a country and we want to pay a fair price we should be running these utilities on a not for profit...