Material scientists at Washington University in St Louis (WUSTL) have developed a bimetallic fuel-cell catalyst that is two to five times more effective than commercial catalysts.
Dr Younan Xia, the James M McKelvey Professor of Biomedical Engineering at WUSTL, led a team of scientists at the university and the Brookhaven National Laboratory in developing the bimetallic catalyst, which is comprised of a palladium core or 'seed' that supports dendritic platinum branches, or arms, that are fixed on the nanostructure.
They synthesised the catalysts by sequentially reducing precursor compounds to palladium and platinum with L-ascorbic acid in an aqueous solution. The catalysts have a high surface area, invaluable for a number of applications besides fuel cells, and are robust and stable.
Xia and his team tested how the catalysts performed in the oxygen reduction reaction process in a fuel cell, which determines how large a current will be generated in an electrochemical system.
They found that their bimetallic nanodendrites, at room temperature, were two to five times more effective than commercially available platinum catalysts.
'There are two ways to make a more effective catalyst,' Xia said. 'One is to control the size, making it smaller, which gives the catalyst a higher specific surface area on a mass basis. Another is to change the arrangement of atoms on the surface. We did both. You can have a square or hexagonal arrangement for the surface atoms. We chose the hexagonal lattice because it's twice as good as the square one for the oxygen reduction reaction.'
Xia added that seeded growth has emerged recently as a good technique for precisely controlling the shape and composition of metallic nanostructures prepared in solutions. And it's the only technique that allowed Xia and his collaborators to come up with their unconventional shape.
Xia and his team are exploring the possibility of adding other noble metals such as gold to the bimetallic catalysts, making them trimetallic. Gold has been shown to oxidise carbon monoxide, making for even more robust catalysts that can resist the poisoning by carbon monoxide - a reduction by-product of some fuels.
'Gold should make the catalysts more stable, durable and robust, giving yet another level of control,' Xia said.
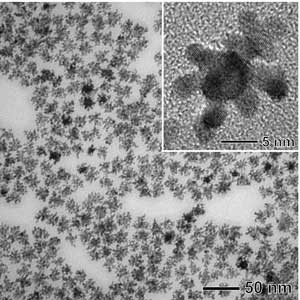
A new catalyst based on dendritic platinum arms grown on palladium nanocrystals has been developed by WUSTL's Younan Xia and his collaborators. Tests have shown that the bimetallic catalyst outperforms commercial catalysts, which could enable a cost-effective fuel-cell technology and ultimately provide cleaner fuels




Poll: Should the UK’s railways be renationalised?
I think that a network inclusive of the vehicles on it would make sense. However it remains to be seen if there is any plan for it to be for the...