Blood test for over 50 cancers gets NHS trial
A new blood test that incorporates AI into its diagnostic techniques to detect more than 50 types of cancer will be trialled by the NHS next year.
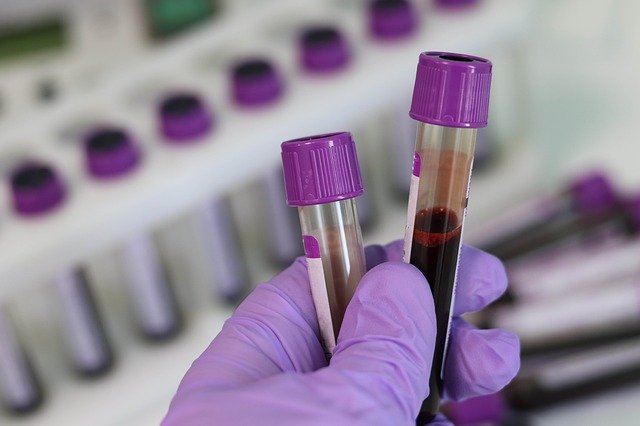
The Galleri test, developed by California-based biotech company Grail, screens for cell-free nucleic acids (cfNAs), small fragments of DNA and RNA that are thought to be cast off by tumours in the body. These cfNAs reflect the genomic profile of the tumours where they originated, allowing not only the potential presence of cancer to be detected, but also its location within the body. Galleri uses artificial intelligence to help distinguish cancerous cfNA’s from the masses of other genomic material floating in the bloodstream.
T-rays help to characterise psoriasis and skin cancer
Grail claims a US clinical study using an earlier iteration of Galleri successfully detected over 50 types of cancer with a low false-positive rate of less than one per cent through a single blood draw. The company’s own modelling indicates the test has the potential to decrease the number of cancers diagnosed at late stage by nearly half, which could reduce the total number of cancer deaths in the UK by approximately one-fifth.
Register now to continue reading
Thanks for visiting The Engineer. You’ve now reached your monthly limit of news stories. Register for free to unlock unlimited access to all of our news coverage, as well as premium content including opinion, in-depth features and special reports.
Benefits of registering
-
In-depth insights and coverage of key emerging trends
-
Unrestricted access to special reports throughout the year
-
Daily technology news delivered straight to your inbox










UK Enters ‘Golden Age of Nuclear’
The delay (nearly 8 years) in getting approval for the Rolls-Royce SMR is most worrying. Signifies a torpid and expensive system that is quite onerous...