Breaking the nanostructure mould
Researchers at the
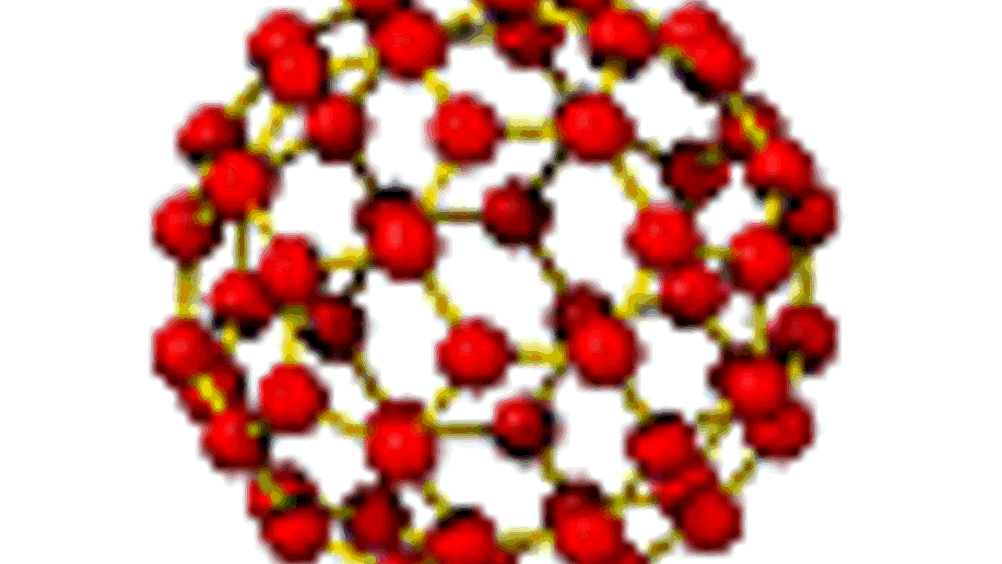
Most conventional methods for the production of porous silicon dioxide nanoparticles suffer from the fact that the growing particles tend to aggregate, making it difficult to achieve a uniform size. The shape of the particles is also difficult to influence.
The
The ‘moulds’ used for the lattice were tiny spheres of the plastic polymethylmethacrylate (PMMA), which assemble themselves through closest packing of spheres into a colloidal crystal. Between the spheres in this structure, there are tiny approximately tetrahedral and octahedral spaces.
Register now to continue reading
Thanks for visiting The Engineer. You’ve now reached your monthly limit of news stories. Register for free to unlock unlimited access to all of our news coverage, as well as premium content including opinion, in-depth features and special reports.
Benefits of registering
-
In-depth insights and coverage of key emerging trends
-
Unrestricted access to special reports throughout the year
-
Daily technology news delivered straight to your inbox










Pipebots Transforming Water Pipe Leak Detection and Repair
Fantastic application.