Carnivorous plant inspires coating that fights biofouling on medical implants
A coating inspired by the Nepenthes pitcher plant has prevented bacteria from attaching to surfaces treated with it, a development that could mitigate against biofouling on implanted medical devices.

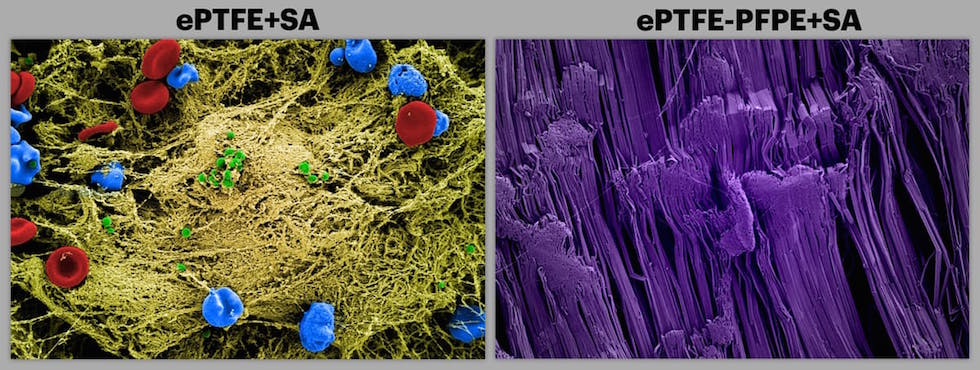
In a report published in Biomaterials, a team of scientists at Beth Israel Deaconess Medical Center (BIDMC), the Wyss Institute for Biologically Inspired Engineering and the John A. Paulson School of Engineering and Applied Sciences (SEAS) at Harvard University has demonstrated that the innovative, ultra-low adhesive coating reduced bacterial adhesion by more than 98 per cent in laboratory tests.
"Device related infections remain a significant problem in medicine, burdening society with millions of dollars in health care costs," said Elliot Chaikof, MD, PhD, chair of the Roberta and Stephen R. Weiner Department of Surgery and Surgeon-in-Chief at BIDMC and an associate faculty member at the Wyss Institute. "Antibiotics alone will not solve this problem. We need to use new approaches to minimize the risk of infection, and this strategy is a very important step in that direction."
The self-healing slippery surface coatings - dubbed 'slippery liquid-infused porous surfaces' (SLIPS) - were developed by Joanna Aizenberg, PhD, a Wyss Institute core faculty member, Professor of Chemistry and Chemical Biology and the Amy Smith Berylson Professor of Materials Science at SEAS at Harvard University.
Register now to continue reading
Thanks for visiting The Engineer. You’ve now reached your monthly limit of news stories. Register for free to unlock unlimited access to all of our news coverage, as well as premium content including opinion, in-depth features and special reports.
Benefits of registering
-
In-depth insights and coverage of key emerging trends
-
Unrestricted access to special reports throughout the year
-
Daily technology news delivered straight to your inbox










National Gas receives funding to develop Gravitricity underground hydrogen storage system
One single rock salt mine - Winsford - has 23 <i>MILLION </i>cubic metres of void and even allowing for 10% of that void set aside for hazardous waste...