Catalysts enhanced with atom scale observations
A new understanding of catalysts at the atomic level could lead to energy-efficient and sustainable catalytic processes for a range of applications.
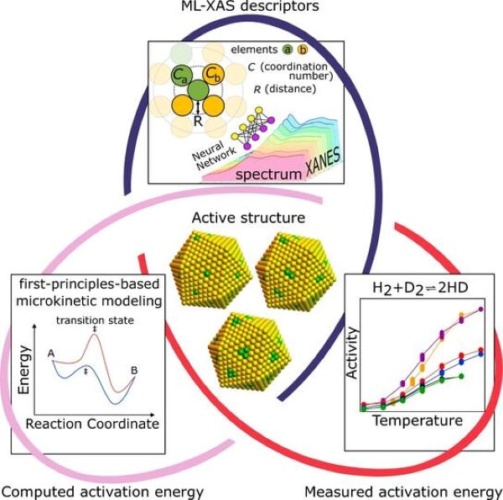
Insights into the reaction pathways and kinetics of catalytic reactions at the atomic scale is critical to designing catalysts for more energy-efficient and sustainable chemical production, particularly multimaterial catalysts that have ever-changing surface structures.
Now, and for the first time, researchers from the Harvard John A. Paulson School of Engineering and Applied Sciences (SEAS), in collaboration with researchers from Stony Brook University, University of Pennsylvania, University of California, Los Angeles, Columbia University, and University of Florida, have an understanding of the evolving structures in a multimaterial catalyst.
The research was done as part of the Integrated Mesoscale Architectures for Sustainable Catalysis (IMASC), an Energy Frontier Research Center funded by the US Department of Energy, headquartered at Harvard. The research is detailed in a paper published in Nature Communications.
“Our multipronged strategy combines reactivity measurements, machine learning-enabled spectroscopic analysis, and kinetic modelling to resolve a long-standing challenge in the field of catalysis - how do we understand the reactive structures in complex and dynamic alloy catalysts at the atomic level,” said Boris Kozinsky, the Thomas D. Cabot Associate Professor of Computational Materials Science at SEAS and co-corresponding author of the paper. “This research allows us to advance catalyst design beyond the trial-and-error approach.”
Register now to continue reading
Thanks for visiting The Engineer. You’ve now reached your monthly limit of news stories. Register for free to unlock unlimited access to all of our news coverage, as well as premium content including opinion, in-depth features and special reports.
Benefits of registering
-
In-depth insights and coverage of key emerging trends
-
Unrestricted access to special reports throughout the year
-
Daily technology news delivered straight to your inbox










National Gas receives funding to develop Gravitricity underground hydrogen storage system
One single rock salt mine - Winsford - has 23 <i>MILLION </i>cubic metres of void and even allowing for 10% of that void set aside for hazardous waste...