But now, researchers at the US Department of Energy’s Pacific Northwest National Laboratory have shown that modifying the battery’s electrolyte solution can significantly improve its performance.
They found that by adding hydrochloric acid to the sulphuric acid typically used in the batteries increased the batteries’ energy storage capacity by 70 per cent and expanded the temperature range in which they operate.
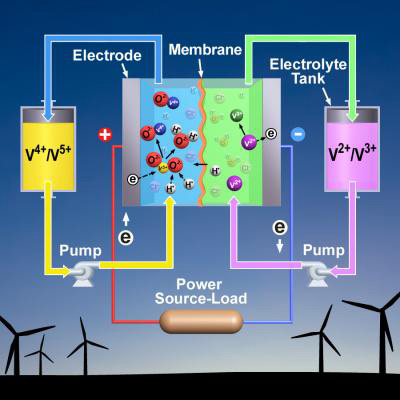
A vanadium battery generates power by pumping liquid from external tanks to the battery’s central stack, or a chamber where the liquids are mixed. The tanks contain electrolytes, which are liquids that conduct electricity. One tank has the positively charged vanadium ion V5+ floating in its electrolyte, while the other tank holds an electrolyte full of a different vanadium ion, V2+. When energy is needed, pumps move the ion-saturated electrolyte from both tanks into the stack, where a chemical reaction causes the ions to change their charge, creating electricity.
To charge the battery, electricity is sent to the vanadium battery’s stack. This causes another reaction that restores the original charge of vanadium ions. The electrolytes with their respective ions are pumped back into their tanks, where they wait until electricity is needed and the cycle is started again.
A battery’s capacity to generate electricity is limited by how many ions it can pack into the electrolyte. Vanadium batteries traditionally use pure sulphuric acid for their electrolyte. But sulphuric acid can only absorb so many vanadium ions.
Another drawback is that sulphuric acid-based vanadium batteries only work between about 50°F and 104°F (10°C to 40°C). Below that temperature range, the ion-infused sulphuric acid crystallises. The larger concern, however, is the battery overheating, which causes an unwanted solid to form and renders the battery useless. To regulate the temperature, air conditioners or circulating cooling water are used, which causes up to 20 per cent energy loss and significantly increases the battery’s operating cost.
Wanting to improve the battery’s performance, Pacific Northwest National Laboratory chemist Liyu Li and his colleagues began searching for a new electrolyte. They found the ideal balance when they mixed six parts of hydrochloric acid with 2.5 parts of sulphuric acid.
Tests showed that the new electrolyte mixture could hold 70 per cent more vanadium ions, making the battery’s electricity capacity 70 per cent higher. The discovery means that smaller tanks can be used to generate the same amount of power as larger tanks filled with the old electrolyte.
The new mixture also allowed the battery to work in both warmer and colder temperatures, between 23°F and 122°F (-5°C to 50°C), greatly reducing the need for costly cooling systems. At room temperature, a battery with the new electrolyte mixture maintained an 87 per cent energy-efficiency rate for 20 days, which is about the same efficiency as the old solution.
’Vanadium redox batteries have been around for more than 20 years, but their use has been limited by a relatively narrow temperature range,’ Li said. ’Something as simple as adjusting the batteries’ electrolyte means they can be used in more places without having to divert power output to regulate heat.’





Comment: How engineering innovations are transforming washing machine accessibility
I wonder if Mr Sawhney has considered a sit-on version, with pedals?