The team, led by engineers at the University of California, Berkeley, and Lawrence Berkeley National Laboratory, has found a way of improving the quality of monolayer semiconductors, a class of atomically thin materials thought to hold promise in the development of transparent LED displays, ultra-high efficiency solar cells, photo detectors and nanoscale transistors.
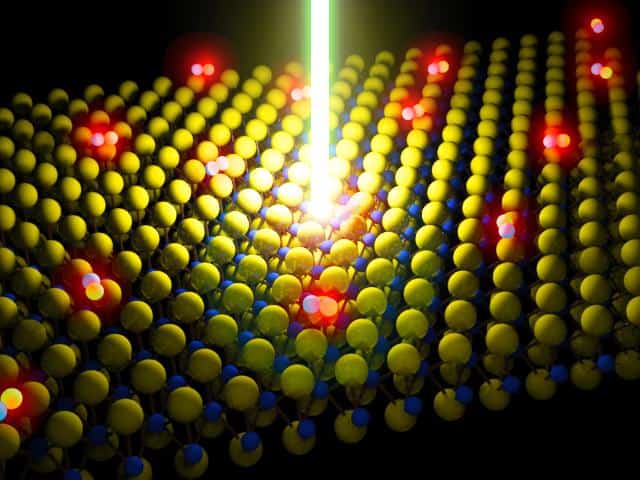
In the past, the performance of this material has been hampered by the fact that it is typically riddled with defects, however the group has found a way to fix these defects through the use of an organic superacid which it claims has led to a 100-fold increase in the material’s photoluminescence quantum yield, a ratio describing the amount of light generated by the material versus the amount of energy put in. The greater the emission of light, the higher the quantum yield and the better the material quality.
The findings, published in Science, opens the door to the practical application of monolayer materials, such as MoS2, in optoelectronic devices and high-performance transistors. MoS2 is a mere seven-tenths of a nanometer thick.
“Traditionally, the thinner the material, the more sensitive it is to defects,” said principal investigator Ali Javey, UC Berkeley professor of electrical engineering and computer sciences and a faculty scientist at Berkeley Lab. “This study presents the first demonstration of an optoelectronically perfect monolayer, which previously had been unheard of in a material this thin.”
The researchers looked to superacids because, by definition, they are solutions with a propensity to “give” protons, often in the form of hydrogen atoms, to other substances. This chemical reaction, called protonation, has the effect of filling in for the missing atoms at the site of defects as well as removing unwanted contaminants stuck on the surface, the researchers said.




Poll: Should the UK’s railways be renationalised?
I think that a network inclusive of the vehicles on it would make sense. However it remains to be seen if there is any plan for it to be for the...