OptiNose attracts $48.5m
Norwegian drug delivery company OptiNose has received $48.5 million to take its breath-actuated nasal delivery device through its final trial before seeking US government approval.

The device, which was developed with Cambridge Consutants, delivers drugs to parts of the nose that standard nasal sprays are unable to reach, while preventing drugs from reaching the lungs.
OptiNose is based on a multi-dose, bi-directional system, which includes a mouthpiece and nasal nozzle. As the patient exhales through the mouthpiece into the device, the airflow will carry spray droplets of the drug into the upper posterior nasal passage beyond the nasal valve to the sinus region.
Helena Kyttari Djupesland, chief executive of OptiNose, explained that when a patient exhales, it lifts a soft pallet at the back mouth that closes off access to the throat and lungs. This means, she said, the drug has nowhere else to go but the nose.
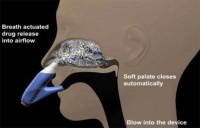
With traditional nasal sprays, Djupesland said, drugs cannot reach the areas they reach with OptiNose. ‘With the application of drugs using normal spray pumps, you feel a wetness and you feel it will run out so you sniff and actually end up swallowing it,’ she said.
Register now to continue reading
Thanks for visiting The Engineer. You’ve now reached your monthly limit of news stories. Register for free to unlock unlimited access to all of our news coverage, as well as premium content including opinion, in-depth features and special reports.
Benefits of registering
-
In-depth insights and coverage of key emerging trends
-
Unrestricted access to special reports throughout the year
-
Daily technology news delivered straight to your inbox










Water Sector Talent Exodus Could Cripple The Sector
Maybe if things are essential for the running of a country and we want to pay a fair price we should be running these utilities on a not for profit...