Power though goo
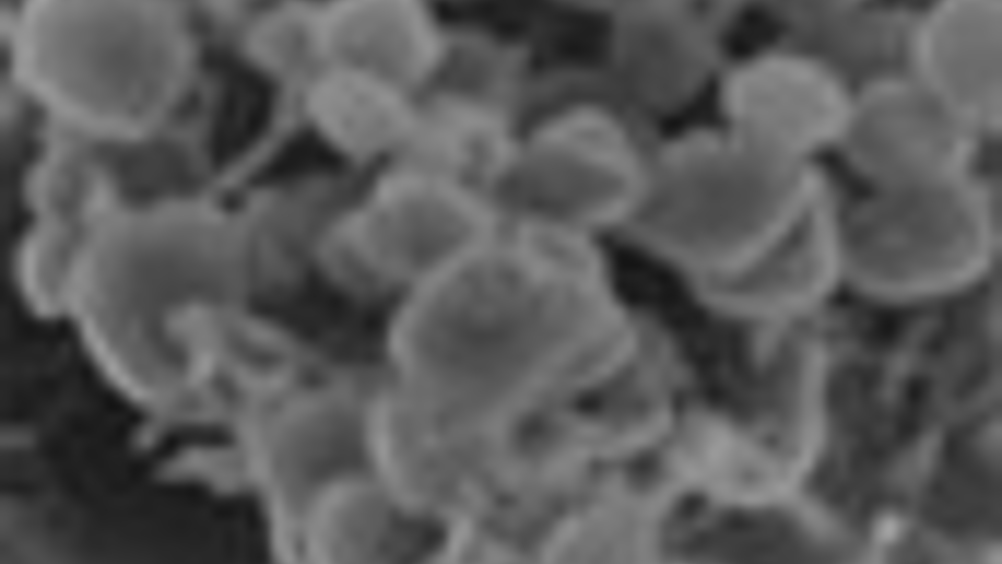
Scientists have boosted the power output of microbial fuel cells more than 10 fold by letting the bacteria congregate in a biofilm.
The research, led by microbiologist Derek Lovley of the
A typical fuel cell converts fuels to electricity without the need for combustion and microbial fuel cells work the same way. They usually comprise two compartments, or cells, which are separated by an electrically insulating membrane. In one compartment, microorganisms pull electrons and protons from some sort of fuel, such as waste organic matter. These protons and electrons are attracted to molecules in the second compartment (usually oxygen) and will move towards those molecules. The protons do this by passing through the membrane. But the electrons can’t go through the membrane and so must travel via an alternate route; a wire or electrode that connects the two compartments. It is this flow of electrons through the electrode that supplies power.
Register now to continue reading
Thanks for visiting The Engineer. You’ve now reached your monthly limit of news stories. Register for free to unlock unlimited access to all of our news coverage, as well as premium content including opinion, in-depth features and special reports.
Benefits of registering
-
In-depth insights and coverage of key emerging trends
-
Unrestricted access to special reports throughout the year
-
Daily technology news delivered straight to your inbox










Water Sector Talent Exodus Could Cripple The Sector
Maybe if things are essential for the running of a country and we want to pay a fair price we should be running these utilities on a not for profit...