Tuneable nanocrystal gel offers applications in energy and defence
A new type of tuneable nanocrystal gel has the potential to be used as thermal camouflage or as a temperature regulator for buildings.
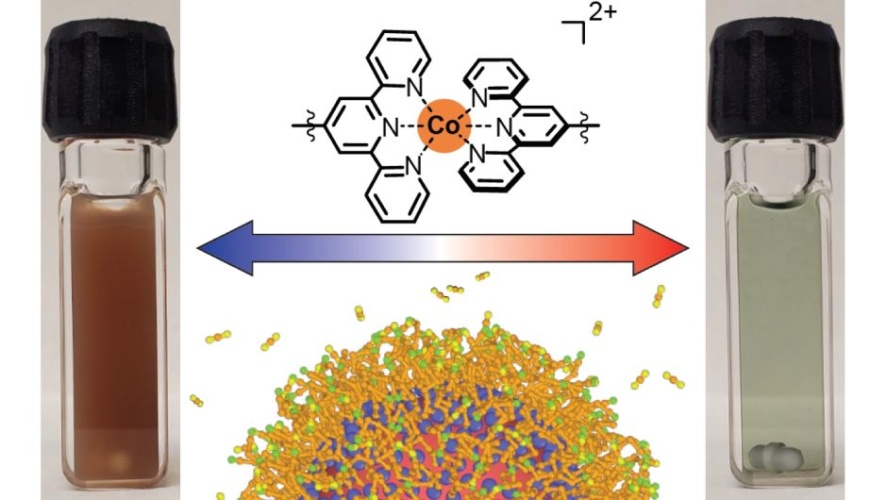
This the claim of a team from The University of Texas at Austin whose new material can be switched between two different states by changing the temperature. This allows it to work as an optical filter, absorbing different frequencies of light depending on whether it’s in a gelled state or not.
In one possible application, the team said the nanocrystal gel could be used on the outside of buildings to control heating or cooling dynamically. This type of optical filter also has applications for defence, particularly for thermal camouflage.
Register now to continue reading
Thanks for visiting The Engineer. You’ve now reached your monthly limit of news stories. Register for free to unlock unlimited access to all of our news coverage, as well as premium content including opinion, in-depth features and special reports.
Benefits of registering
-
In-depth insights and coverage of key emerging trends
-
Unrestricted access to special reports throughout the year
-
Daily technology news delivered straight to your inbox










Water Sector Talent Exodus Could Cripple The Sector
Maybe if things are essential for the running of a country and we want to pay a fair price we should be running these utilities on a not for profit...