Heart patient Phil O’Donoghue is taking part in the study, which is funded with £1.8m from the British Heart Foundation (BHF). Over 2,500 patients are due to be recruited across the UK in the next three years.
Heart failure affects over 900,000 people in the UK, with around 200,000 new cases diagnosed each year at an estimated cost of £2bn to the NHS.
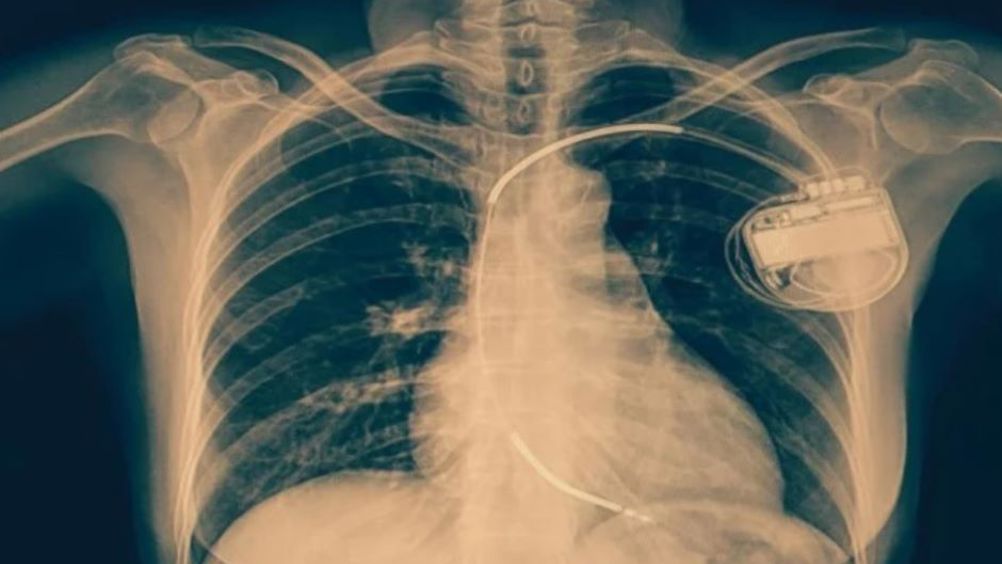
ICDs are small devices that are routinely fitted in the chests of patients with heart failure to stop abnormal rhythms and treat cardiac arrest by delivering an electric shock to the heart.
The study is being led by consultant cardiologists Dr Andrew Flett and Professor Nick Curzen from University Hospital Southampton (UHS) and is co-ordinated by the NIHR Southampton Clinical Trials Unit.
“The current guidelines look at how well the heart is pumping to decide which patients should get a defibrillator,” Dr Flett said in a statement. “But for many patients who go through the procedure of having a defibrillator fitted, they will never actually see the device triggered and may not need it. We therefore want to find a better way to assess which patients will truly benefit from one of these devices.
Dr Flett continued: “There is evidence that scar tissue in the heart muscle may be the cause of dangerous heart rhythms for patients with NICM [non-ischemic cardiomyopathy]. This will be the first ever trial to look at whether the presence of scar tissue can predict who should be fitted with an ICD.”
MORE FROM MEDICAL & HEALTHCARE
An MRI scan will be used to detect the presence of scar tissue in the heart and those patients will be invited into the trial.
“Participants are randomly allocated to one of two trial arms,” said Prof Curzen. “Half will be fitted with an implantable defibrillator. The others will be fitted with an implantable loop recorder [ILR], a device which monitors heart activity so that the team can review any abnormal rhythms, but which does not shock the heart.”
O’Donoghue, 53, suffers from NICM, a common type of heart failure which can lead to abnormal heart rhythms, and sudden cardiac arrests are a possible cause of death in these patients. He is in the defibrillator arm and was the first patient enrolled in the trial to have the device fitted at University Hospital Southampton.
He said: “Having the defibrillator doesn’t bother me, it’s just one of those things. If it goes off, it goes off. And if it doesn’t, then great. But if the trial can show one way or another whether they should be used, and move treatments forward, then it’s obviously a benefit.”
Professor Sir Nilesh Samani, BHF’s medical director, said: “ICDs are crucial devices to treat sudden cardiac arrest and save lives. But it is important that we continue to establish exactly which patients need them, so that people who are unlikely to benefit do not have to undergo invasive procedures unnecessarily. The…trial will test whether the presence of scar tissue in the heart predicts who will benefit most from having an ICD.”
The trial is currently open to patients at five hospitals in Southampton, Portsmouth, Aberdeen, East Kent, and Barts in London, with a further 30 sites to open in the coming months.
“The ultimate goal of any research is to improve treatment for our patients,” said Dr Flett. “The…trial will inform UK and international guidelines for the treatment of heart failure.”










Comment: The UK is closer to deindustrialisation than reindustrialisation
"..have been years in the making" and are embedded in the actors - thus making it difficult for UK industry to move on and develop and apply...