Wearable sensor monitors biomolecules in deep tissue
Engineers in the US claim to have developed an electronic wearable sensor that can monitor biomolecules in deep tissues, including haemoglobin.
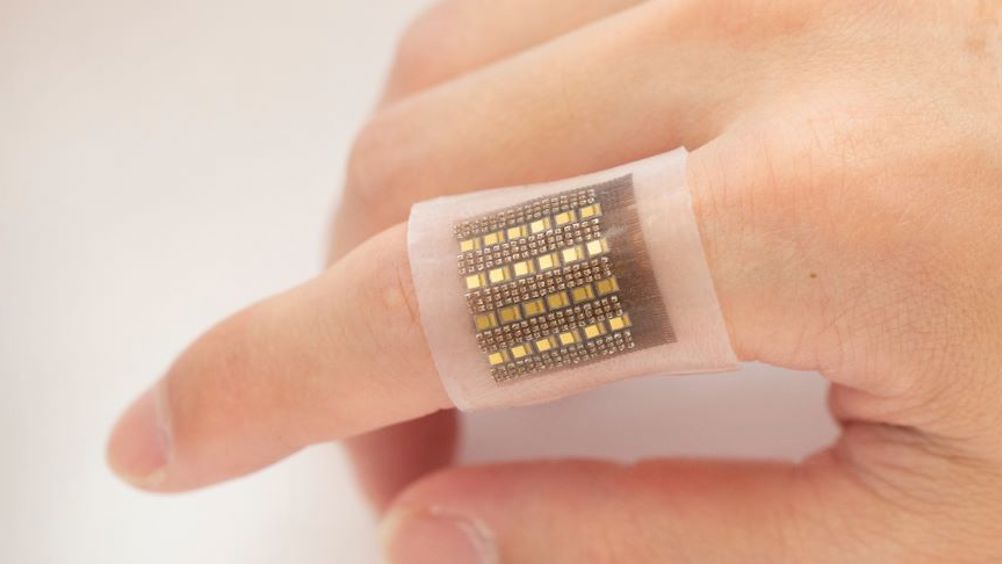
According to the team at University of California San Diego, its skin patch could give medical professionals unprecedented access to crucial information that could help spot life-threatening conditions such as malignant tumours, organ dysfunction, cerebral or gut haemorrhages and more.
“The amount and location of haemoglobin in the body provide critical information about blood perfusion or accumulation in specific locations. Our device shows great potential in close monitoring of high-risk groups, enabling timely interventions at urgent moments,” said Sheng Xu, a professor of nanoengineering at UC San Diego and corresponding author of the study.
Low blood perfusion inside the body may cause severe organ dysfunctions and is associated with a range of ailments, including heart attacks and vascular diseases of the extremities.
At the same time, abnormal blood accumulation in areas such as in the brain, abdomen or cysts can indicate cerebral or visceral haemorrhage or malignant tumours. Continuous monitoring can aid diagnosis of these conditions and help facilitate timely and potentially life-saving interventions.
Register now to continue reading
Thanks for visiting The Engineer. You’ve now reached your monthly limit of news stories. Register for free to unlock unlimited access to all of our news coverage, as well as premium content including opinion, in-depth features and special reports.
Benefits of registering
-
In-depth insights and coverage of key emerging trends
-
Unrestricted access to special reports throughout the year
-
Daily technology news delivered straight to your inbox










Water Sector Talent Exodus Could Cripple The Sector
Maybe if things are essential for the running of a country and we want to pay a fair price we should be running these utilities on a not for profit...