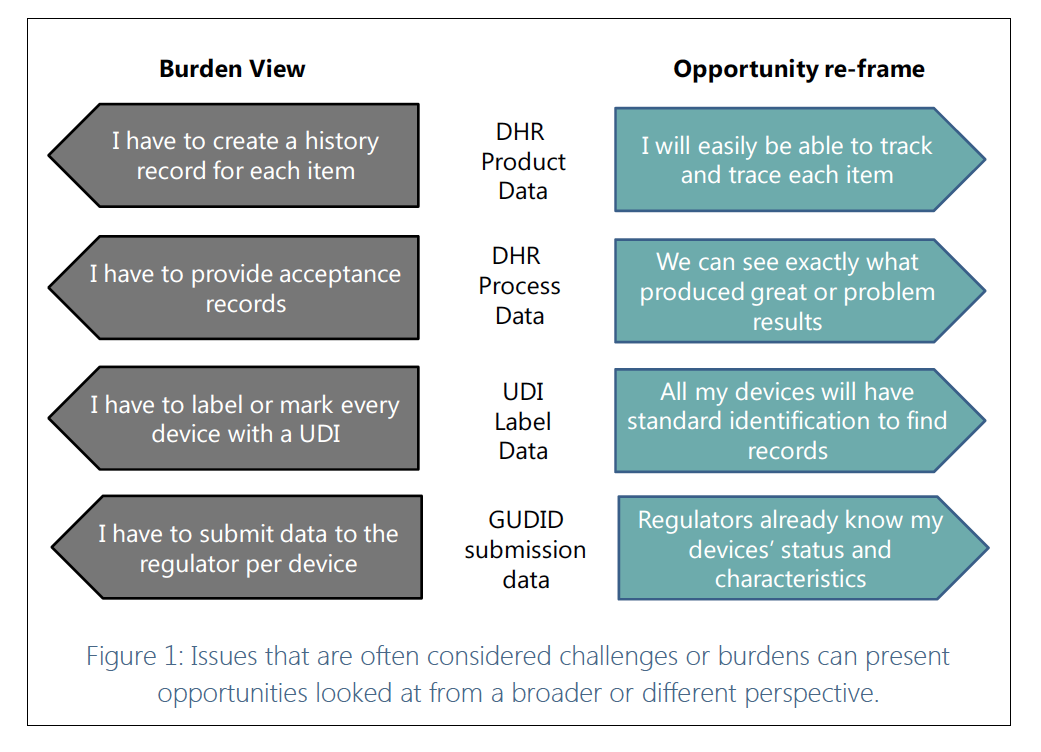
Medical device manufacturers regularly groan about the cost and burden of regulatory compliance. With regulations changing every day around the world, paper-based or disjointed information systems cannot keep up. That is what most companies have, and they will struggle to comply without a hit to profits.
However, this need not be the reality. Companies can, rather than simply viewing traceability requirements as a necessary evil, re-frame traceability to find the opportunities it offers the business.
In fact, medical device companies can and must re-frame to survive. Given that some have seen the opportunity in end-to-end data flows and are thriving, you might think it’s obvious.










Simulations show Optimal Design for Bladeless Wind Turbines
"an 80cm mast" Really? I'm short but that's only half my height! Do they mean 800cm?