Enzymes generate ammonia at room temperature
University of Utah researchers have used enzymes to generate ammonia at room temperature, a process that also generates a small electrical current.

The method is published in Angewandte Chemie International Edition.
Professor Shelley Minteer and postdoctoral student Ross Milton have only been able to produce small quantities of ammonia so far, but their method could lead to a less energy-intensive source of ammonia, which is used globally as a fertilizer.
"It's a spontaneous process, so rather than having to put energy in, it's actually generating its own electricity," Minteer said in a statement.
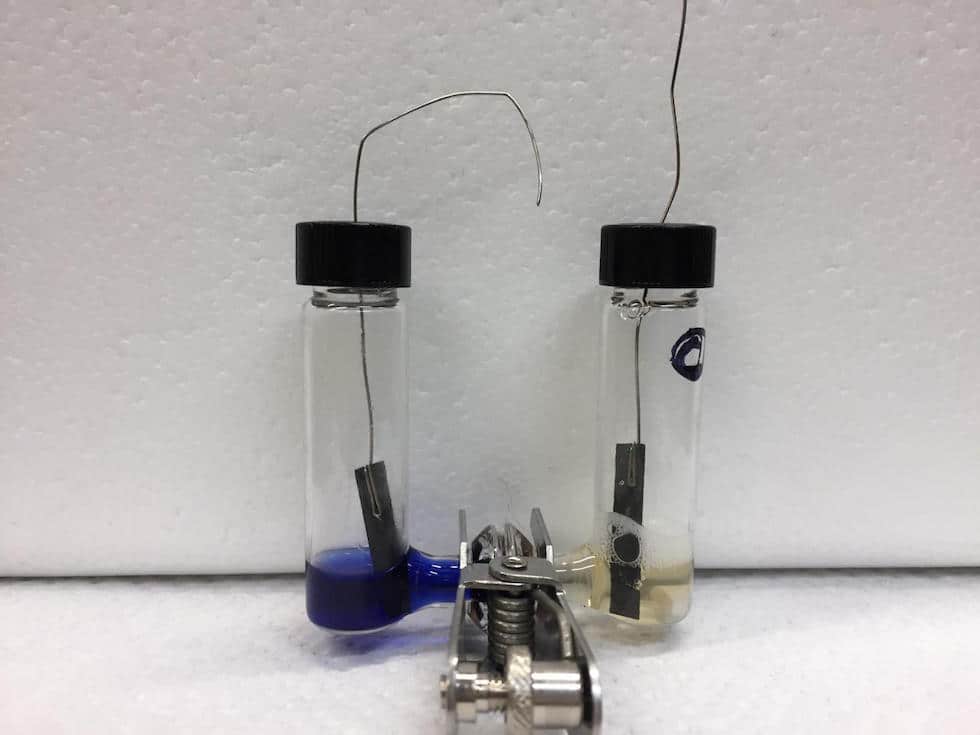
To make ammonia, which consists of one nitrogen atom and three hydrogen atoms, chemists break the bond that holds two nitrogen atoms together, and then reduce the nitrogen, or add electrons and protons to it in the form of hydrogen.
Ammonia is produced on an industrial scale using the Haber-Bosch process, where hydrogen and nitrogen is pumped over beds of metal catalysts at pressures up to 250 times atmospheric pressure and temperatures up to 500 degrees Celsius. The process currently produces nearly 500 million tons of ammonia every year.
Register now to continue reading
Thanks for visiting The Engineer. You’ve now reached your monthly limit of news stories. Register for free to unlock unlimited access to all of our news coverage, as well as premium content including opinion, in-depth features and special reports.
Benefits of registering
-
In-depth insights and coverage of key emerging trends
-
Unrestricted access to special reports throughout the year
-
Daily technology news delivered straight to your inbox










Water Sector Talent Exodus Could Cripple The Sector
Maybe if things are essential for the running of a country and we want to pay a fair price we should be running these utilities on a not for profit...