Supercapacitors are being explored as a possible alternative to the lithium-ion batteries that power everything from smartphones to electric vehicles (EVs). Lithium batteries can require a long time to charge, tend to start degrading quickly, and often fail before reaching 1,500 cycles. Supercapacitors can be charged quickly, but one that held as much energy as a lithium-ion battery would need to be extremely large.
To address the issue, engineers are now looking to nanomaterials. The research, described in the journal ACS Nano, saw the UCF team applying newly discovered two-dimensional materials just a few atoms thick to supercapacitors. They used 2D transition-metal dichalcogenides (TMDs) integrated with an array of one-dimensional nanowires.
According to the paper’s abstract, these hybrid supercapacitors outperform previously developed stand-alone 2D TMD-based supercapacitors, with their structural robustness delivering more than 30,000 charge-discharge cycles with no drop in performance. Previous researchers have attempted similar techniques using graphene and other 2D materials, but success has been limited.
"There have been problems in the way people incorporate these two-dimensional materials into the existing systems - that's been a bottleneck in the field,” said principal investigator Yeonwoong Jung, an assistant professor at UCF’s NanoScience Technology Centre and Materials Science & Engineering Department.
“We developed a simple chemical synthesis approach so we can very nicely integrate the existing materials with the two-dimensional materials."
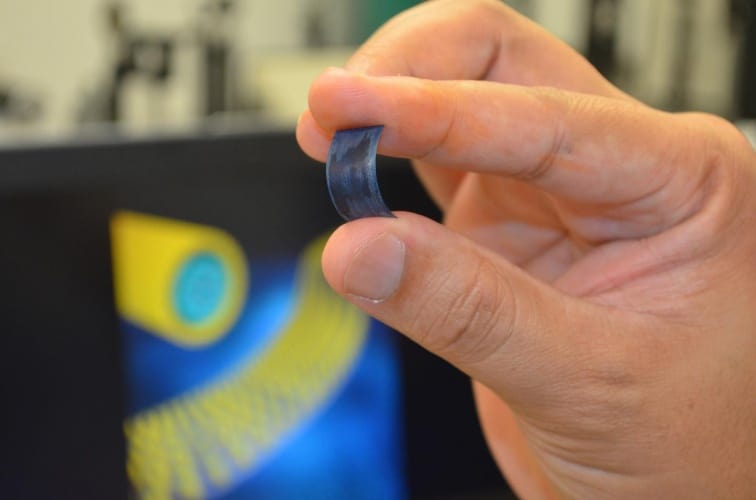
Although not yet ready for commercialisation, the technology holds promise for a wide range of electronics devices, as well as EVs that require a sudden burst of power. The materials are also flexible, giving them potential applications in wearable tech.
"For small electronic devices, our materials are surpassing the conventional ones worldwide in terms of energy density, power density and cyclic stability," said Nitin Choudhary, a postdoctoral associate at UCF who conducted much of the research.
"If they were to replace the batteries with these supercapacitors, you could charge your mobile phone in a few seconds and you wouldn't need to charge it again for over a week."




Nanogenerator consumes CO2 to generate electricity
Nice to see my my views being backed up by no less a figure than Sabine Hossenfelder https://youtu.be/QoJzs4fA4fo