As electric power becomes more important for everything from ubiquitous computing to transport, researchers are increasingly looking for ways to avoid some of the drawbacks of current electricity storage devices. Whether they are batteries, which release a steady stream of electric current, or supercapacitors, which release a sharper burst of charge, storage devices depend on conductive electrolyte fluids to carry charge between their electrodes. Susceptible to leakage and often flammable, these fluids have been behind many of the reported problems with batteries in recent years, including fires on board aircraft and exploding tablet computers (the later being caused by short-circuiting inside miniaturised batteries).
A team at Drexel University in Pennsylvania is now claiming to have made progress towards solving this problem, by constructing an electrode material resembling a porous mat of conductive carbon nanofibres that is saturated with a viscous ion-rich gel electrolyte. Not only is this combination free of flammable solvents, but it is also claimed to be more durable and lighter than comparable devices currently in use, and to have better energy storage characteristics and charge-discharge lifespan.
In the journal ACS Applied Materials and Interfaces, the Drexel team, led by Dr Vibha Kalra, describes how it used electrospinning — extruding a carbon precursor polymer solution through a rotating electric field — to make a mat of carbon fibres with a texture resembling candyfloss at a molecular level and with a very high specific surface area (2282m2 per gramme). They then introduced their electrolyte, a material known as an ionogel consisting of the organic liquid 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide made into a gel with a methylcellulose polymer matrix. The electrode fibre material absorbs the gel, so that the charge-carrying ions in the electrolyte make contact across the large surface area of the electrode, which is the key to the greater capacity of the material to store and conduct charge.
Replacing the liquid electrolyte solution of a typical battery or supercapacitor with a gel means that "we have completely eliminated the component that can catch fire in these devices," Kalra said.
Moreover, she added, the structure of the electrode is very different from a typical electrode, which improves its performance. "State of the art electrodes are composed of fine powders that need to be blended with binding agents and made into a slurry, which is then applied into the device. These binders add dead weight to the device, as they are not conductive materials, and they actually hinder its performance," Kalra explained. "Our electrodes are freestanding, thus eliminating the need for binders, whose processing can account for as much as 20 per cent of the cost of manufacturing an electrode."
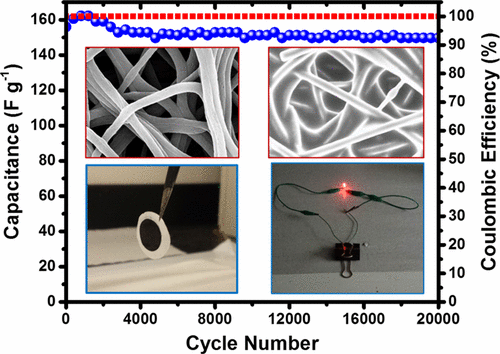
The Drexel team has used the electrode combination to make capacitors, obtaining a capacitance of 153F/g and energy density of 65Wh/kg, with capacitance fading by 4 per cent over 20,000 charge-discharge cycles. Their next goal is to construct and test solid-state batteries and explore whether the technology could be used in ‘smart fabrics’.
See how the team spun the carbon fibre electrode in this video:
https://giphy.com/gifs/batteries-electrode-carbon-fiber-l378kDRm9oxNfLc0U





Poll: Should the UK’s railways be renationalised?
Rail passenger numbers declined from 1.27 million in 1946 to 735,000 in 1994 a fall of 42% over 49 years. In 2019 the last pre-Covid year the number...