Orteq develops biodegradable scaffold for meniscus surgery
Doctors can give athletes who undergo meniscus surgery the ability to return to their active life faster with much less pain using a biodegradable scaffold in the knee.
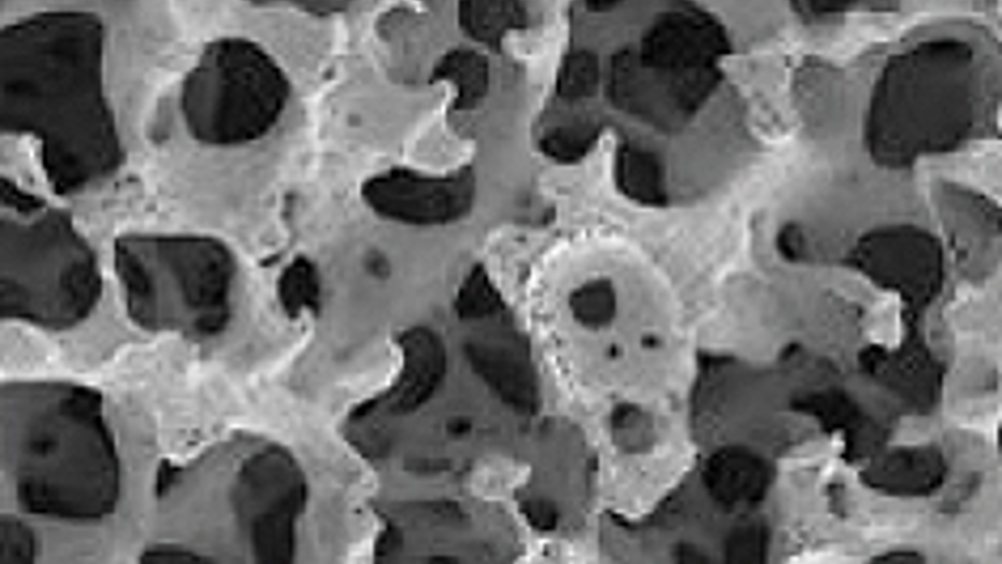
Orthopaedic medical technology company Orteq claims that these are the results so far of patients implanted with its scaffold product dubbed Actifit.
The device has been designed specifically for meniscus surgery, the most popular orthopaedic procedure. According to Orteq, surgeons treat one-and-a-half million meniscal injuries each year in Europe and the US alone.
In these procedures, surgeons try and remove as little meniscus tissue as possible. This is because the meniscus, which is made up of two half-moon-shaped fibrocartilaginous structures in the knee, acts as shock absorbers. Any lost tissue will affect load distribution in the knee and this causes pain and cartilage damage.
Orteq’s scaffold, which is made of a biodegradable and biocompatible polyurethane, is described as a porous cellular matrix. After damaged meniscus tissue is removed, the scaffold is put in place. Over time, cells and blood vessels will grow into the porous material and the scaffold will start filling up with tissue.
Register now to continue reading
Thanks for visiting The Engineer. You’ve now reached your monthly limit of news stories. Register for free to unlock unlimited access to all of our news coverage, as well as premium content including opinion, in-depth features and special reports.
Benefits of registering
-
In-depth insights and coverage of key emerging trends
-
Unrestricted access to special reports throughout the year
-
Daily technology news delivered straight to your inbox










Water Sector Talent Exodus Could Cripple The Sector
Maybe if things are essential for the running of a country and we want to pay a fair price we should be running these utilities on a not for profit...