A new material that sports a large ionic conductivity could help researchers build more efficient fuel cells.
The structure of the material, a super-lattice developed by researchers at Spain's Universidad Complutense de Madrid and Universidad Politécnica de Madrid, was recently characterised at the US Department of Energy's Oak Ridge National Laboratory (ORNL).
The analysis was done with ORNL's 300kV Z-contrast scanning transmission electron microscope which produced images that showed the crystal structure that accounts for the material's conductivity.
Maria Varela of ORNL's Materials Science and Technology Division, who analysed the material's structure with senior researcher, Stephen Pennycook, said: 'We can see the strained, yet still ordered interface structure that opens up a wide pathway for ions to be conducted.'
In a solid oxide fuel cell, solid electrolytes allow oxygen ions to travel from cathode to anode. However, existing materials have not provided atomic voids large enough to easily accommodate the path of the conducted ions.
Varela added: 'The new layered material solves this problem by combining two materials with very different crystal structures. The mismatch triggers a distortion of the atomic arrangement at their interface and creates a pathway through which ions can easily travel.'
Other fuel cell materials force ions to travel through tight pathways with few spaces for the ions to occupy, slowing their progress. Rather than forcing the ions to jump from hole to hole, the new material has 'lots of vacant spaces to be occupied,' according to Varela, so the ions can travel much more quickly.
Unlike previous fuel cell materials, which have to achieve high temperatures to conduct ions, the new material maintains ionic conductivity near room temperatures. High temperatures have been a major issue for developers of fuel cell technology.
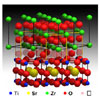
The molecular model of the ion-conducting material shows that numerous vacancies at the interface between the two layers create an open pathway through which ions can travel




Poll: Should the UK’s railways be renationalised?
The term innovation is bandied about in relation to rail almost as a mantra. Everything has to be innovative. There is precious little evidence of...