
If you’re over 85, you’re no longer simply classified as ’old’. You are, instead, part of an elite group that the Office for National Statistics (ONS) now refers to as ’the oldest old’. Last year, the ONS estimated that 1.3 million people in the UK belonged to this category and that this figure would double over the next 25 years.
In Britain, around 40 per cent of over 65s suffer from a long-standing illness or disability, increasing to 69 per cent for those over 85
Give this statistic to a politician and they’ll probably spin it into positive news - we’re living longer and enjoying a higher standard of living. But the reality is that the increased quantity of life hasn’t led to an improvement in quality. In Britain, around 40 per cent of over 65s suffer from a long-standing illness or disability, increasing to 69 per cent for those over 85.
One man, however, is on a mission to change all of that. Prof John Fisher, a mechanical engineer and deputy vice-chancellor of Leeds University, believes that those living to the age of 100 could have the body and fitness level of a 50 year old if tissue-regeneration technology proves successful. According to his estimations, that would be half of all babies born in the UK today.
’You really start old age at around 40, when your bones and joints start to degenerate,’ said Fisher. ’The problem is that 40-60 year olds now want to remain very active, but actually their bodies are degrading. Early intervention would give them some time. Whether this intervention would last for 50 years in the musculo-skeletal system, we don’t know yet.’
“You really start old age at around 40, when your bones and joints start to degenerate.”
Fisher’s flagship programme, ’50 active years after 50’, aims to find this out. Over the next five years, his team at Leeds will spend £50m developing new medical devices and therapies that could allow people to be as active during their second half century as they were in their first. Work is currently focused on repairing the joints, spine, teeth, heart and circulation, but the implications for the rest of the body could be huge.
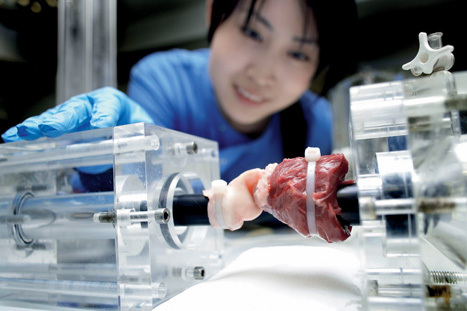
So too could the commercial opportunities, with interest now being directed at Fisher’s work from the likes of China. As head of the Biomaterials and Tissue Engineering Centre of Industrial Collaboration, Fisher is keen to emphasise that his work is not blue-skies research, but will have long-term social and economic benefits. The challenge of an ageing population, he believes, is far too great not to create a targeted solution.
’My academic career hasn’t been very conventional,’ said Fisher. ’I had 10-12 years outside academia, where I initially worked in the automotive, defence and healthcare sectors, but it has allowed me to see the impact of research and technology in a different way. So, when I do research, I try to establish what the challenge is - not from the academic perspective, but from how the research might impact on society.’
The university’s Institute of Medical and Biological Engineering has developed a ceramic-on-metal hip joint that, unlike a metal hip joint, can last the 100 million steps that a 50 year old will take on their way to their 100th birthday. The joint was licensed to Johnson and Johnson in 2007, and is now being marketed globally. Fisher is also working on other interventions, including spinal-disc and knee-joint replacement.
Alongside this, a more radical area of research is being undertaken to provide pensioners with own-grown tissues that could replace ageing body parts. The concept is to use biological scaffolds derived from human or animal tissues for regenerative interventions in the cardiovascular and musculoskeletal systems. A spin-out company from Leeds, Tissue Regenix, has patented the process, which removes cells from tissue in order to be accepted into the body when implanted.
’Everyone else is trying to create this tissue scaffold from synthetic chemistry or by building it from the bottom up,’ said Fisher. ’As an engineer, I just thought: let’s think about it in a very different way. Let’s try and create it from the top down. So you use a tissue structure from an animal, remove the things that make that essentially incompatible with a human and what you’re left with is the architecture to deliver what you want. It’s kind of a reverse engineering and it’s that simple, but it works.’
The body’s own cells can attach to the biological architecture, removing the need for patients to take anti-rejection drugs. The approach has the potential to replace every piece of tissue in the musculoskeletal and cardiovascular system. At the moment, it is being used for a handful of applications, including patches for blood vessels and heart valves, but Fisher believes there are another 10 to 12 products down the line.
’It’s really motivating to see your research actually taken into the patient,’ he said. ’We’ve now licensed it to the NHS national tissue service, which is using it for modifying donor tissue. The potential in this is very exciting.’ Engineers are facing a very different set of challenges, but Fisher believes that the UK is taking the right approach. ’Regenerative medicine will touch all of our lives,’ he said. ’We’ll just have to wait and see exactly how far it can go.’
Q&A Tissue issues
Why did you enter the medical field?
I worked outside medical engineering for a number of years. I graduated and began as a trainee graduate engineer in industry, and I worked in different industry sectors - automotive, defence… While they were technically challenging jobs, they weren’t really fulfilling in terms of what eventually we were delivering. So I then started working in the NHS in a role that was more bioengineering relating to patient care. That led my interests more into research and so I then came back into it and started to work more closely with universities. I definitely find medical engineering more personally rewarding.
Why is China such a big target market for your research?
In China, these types of healthcare technologies are growing at a rate of 40 per cent per year, whereas, in the Western world, they are growing at 10 per cent. That is partly because health and social-care standards are increasing. So they have around 350 million people, about the size of the US, on the Eastern Seaboard, whose standard of living and healthcare is beginning to approach that of Western standards. But then they have a second tier of population, which is another billion who won’t, at the moment, even have access to this type of intervention. So there is a substantial market growth going forward.
What challenges have you come up against working in such a multidisciplinary field?
I wouldn’t necessarily call them challenges. I see them as opportunities. Lots of medical-engineering departments are really about using conventional disciplines of physics, physical science and maths, to look at a medical situation. We’ve tried to recognise that the input is much more diverse than that. We have input from biology, as well as input from physical sciences. The problems are multidisciplinary, so you need to have a multidisciplinary approach.
How does your biological scaffold compare in costs with tissue engineered outside the body?
The ability to control the product is much greater and so our approach can cost about 10 times less in development and 10 times less in product expense. I think that is absolutely fundamental because, at the moment, nobody has been successful in making money out of a cell-based therapy. To engineer a process that reliably produces a tissue with cells that are grown outside the body would cost around £10,000 to £20,000 per treatment. Ours would cost around £1,000 per treatment or less.
What is the ideal environment to make innovation flourish?
I think innovation flourishes in a less-constrained environment. So it flourishes in universities and in small companies - it’s really hard to do this stuff in big organisations. They can be very risk averse, they need very robust investment and return plans, and many of them already have existing technology that they can make money out of, so it’s got to be really good to displace it. What they would rather do is let someone else take the risk and then acquire it when the risk is lower. The public sector can’t always be innovative, but the way we work means we can take on some of the risk in pushing research forward.
Biography
Prof John Fisher
Education
1976 BSc (Hons) First Class in physics at Birmingham University
1977 Postgraduate Certificate in design and manufacture at Preston Polytechnic
1986 PhD in bioengineering at Glasgow University
Career
1976 Became at trainee engineer at British Leyland Truck and Bus Division
1977 Employed by Ferranti Laser Systems as a development engineer
1986 First job in the medical field as principal scientist at the West of Scotland Health Board
1988 Became a lecturer in biomedical engineering and biotribology at Leeds University
1997 Promoted to dean of research in the faculty of engineering
2006 Appointed deputy vice-chancellor and founded spin-out group Tissue Regenix
2011 Received a CBE for services to biomedical engineering





Nanogenerator consumes CO2 to generate electricity
Nice to see my my views being backed up by no less a figure than Sabine Hossenfelder https://youtu.be/QoJzs4fA4fo