Collaborate to Innovate 2018
Category: Healthcare & Medical
Winner: Universal Plasma
Partners: NIRI, NHSBT, Macopharma
Our winner in the healthcare & medical category combines textiles and chemistry innovation in a project that could transform transfusion services and help save lives. Now known as Universal Plasma and spearheaded by NHS Blood and Transplant (NHSBT), the project began life as Sanguis, a collaboration led by Nonwovens Innovation & Research Institute (NIRI), a Leeds-based company that develops innovative textiles products and prototypes for a variety of sectors. Working in partnership with biomed company Macopharma and NHSBT, NIRI developed a new type of filter that removes ABO antibodies from donated human plasma, creating universal plasma (UP) that can be used to treat anyone. NHSBT is now leading the next phase of the project, testing to ensure the filter meets the clinical requirements and patent safety, while Macopharma and NIRI are exploring how the technology can be scaled for commercialisation.
Ordinarily, a patient’s blood group must match that of the donor in order for blood and plasma transfusions to be carried out safely. If the wrong type of plasma is given to a patient, the conflicting antibodies can cause haemolysis (rupture of red blood cells) which can result in death. Group AB plasma can be given to anyone, but just four per cent of the UK population has this blood type, meaning its supply is constantly under pressure.
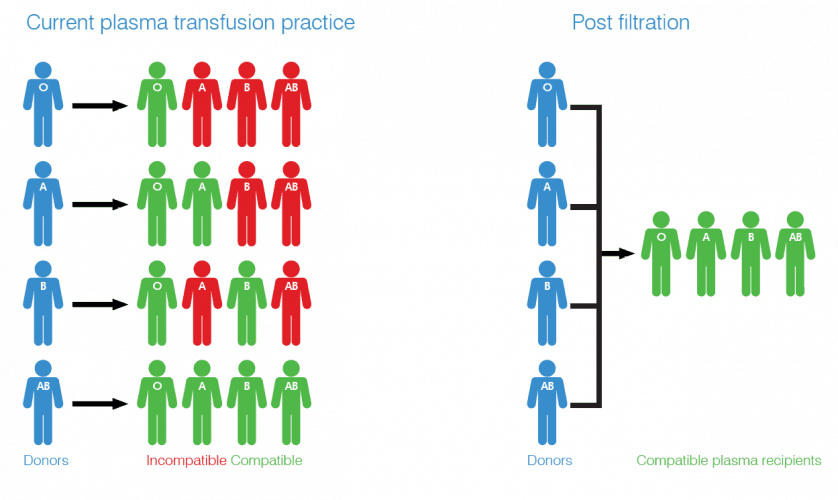
As the only currently available universal plasma, AB is in high demand in emergency and military scenarios. When seconds can be the difference between life and death, it affords medics the luxury of not grouping the patient’s blood before treatment. However, AB’s relative scarcity has prompted the search for an alternative and Universal Plasma could present an economically viable and scalable source. According to Ross Ward, new business development manager at NIRI, the new fabric-based immunoadsorption filter is at the core of the project’s innovation.
“We use an artificial antigen bound to a nonwoven substrate,” Ward told The Engineer. “This artificial antigen is designed to bind the antibodies like a lock and key mechanism. The plasma runs through the filter, antibodies within the plasma are bound to the filter, resulting in a plasma with antibodies removed.”
The technology that NIRI uses to graft these antigens is a new proprietary process. Though the company is unable to share too many details, it essentially involves covalently binding the artificial antigen to fibre surfaces within the nonwoven using chemical reactions. The resulting high-porosity filter can remove ABO antibodies from donated human plasma, converting 300ml into UP in approximately 10 minutes. The UP can then be used to treat anyone, regardless of blood type, as there are minimal antibodies present to react with those in their own plasma.
Though the concept originated with NIRI, the company knew that collaboration would be key to refining the product and testing it in the field. Working with Macopharma, NIRI designed the filter to ensure it was compatible with the biomed company’s process for manufacturing blood packs. NHSBT, which supplies blood across England, also joined the consortium, which was supported by Innovate UK.
“We conducted initial feasibility studies to ensure we were able to bind the artificial antigen and prove the concept,” Ward explained. “Through the project, we worked with Macopharma and NHSBT to develop that technology further.
“NHSBT tested the technology to ensure the filtered plasma remained efficacious and free of active ingredient contamination. Macopharma assisted with developing the technology into a large-scale commercial product.”
Worldwide, more than 7 million units of therapeutic plasma are used each year, with a market value in excess of £350m. The UK represents £20m of that, and the Universal Plasma consortium envisages significant adoption of its filter here, particularly after the clinical and cost benefits become clear. To ensure the product can penetrate the global market, the plan is to offer the technology as an ‘add-on’ rather than integrated directly into blood collection sets. This will allow the filter to be used with any type of blood pack. NIRI is now working with both Macopharma and NHSBT to scale up the process so that it can be deployed en masse.
“The method of producing the filter and binding the artificial antigen to the fibres in the nonwoven is proven,” said Ward. “It works. However, clinical testing, and being able to up-scale into an industrial process, is our current focus. By working in close collaboration with NHSBT and Macopharma we believe we will have a regulatory approved commercialised product within the next five years.”
The runners-up
CHIRON – Designability, Bristol Robotics Laboratory (UWE), Shadow Robot Company, Telemetry Associates Limited, Three Sisters Care Ltd, Smart Homes & Building Alliance (SH&BA)
A collaboration to design the care robots of the future, focusing on dignity and independence
EDEN2020 – Imperial College London, University Medical Center Groningen, Politecnico di Milano, Renishaw, Technische Universität München, Universita' di Milano, The San Raffaele Hospital of Universita Vita-Salute San Raffaele, Xograph Healthcare ltd
Multifaceted EU-backed project to deliver tailored neurosurgery treatment
Wireless wearable targets cells in fight against cancer – University of Nottingham, University of Melbourne, Lawrence Livermore National Laboratory, University of Minnesota, Surescreen Diagnostics
Developing new technology to broaden the use of bioelectronics-based therapeutics
Multi-functional Bioactive Medical Devices for Musculoskeletal Regeneration - The University of Sheffield, Ceramisys Ltd
A new generation of antimicrobial bone graft substitutes to treat or prevent deep bone infection
SLIPS - University of Leeds, NHS Leeds Teaching Hospitals Trust Steeper Group PLC
Innovative tactile sensing technology for assessment and treatment of diabetic foot disease





Plasma technique converts landfill methane to jet fuel
Trevor; there are still many landfill sites in the UK where the methane is not captured but not only that, where there is protein waste in them, they...