Hydrogen, which some claim will be the dominant automotive fuel of the future, is usually produced by separating water with electrical power, a process which typically relies on platinum or other expensive noble metals to help the water-splitting process.
However, according to a report in Nature Materials, a new hydrogen-making catalyst containing phosphorus and sulphur and cobalt, could offer a highly effective, lower cost alternative to existing catalysts.
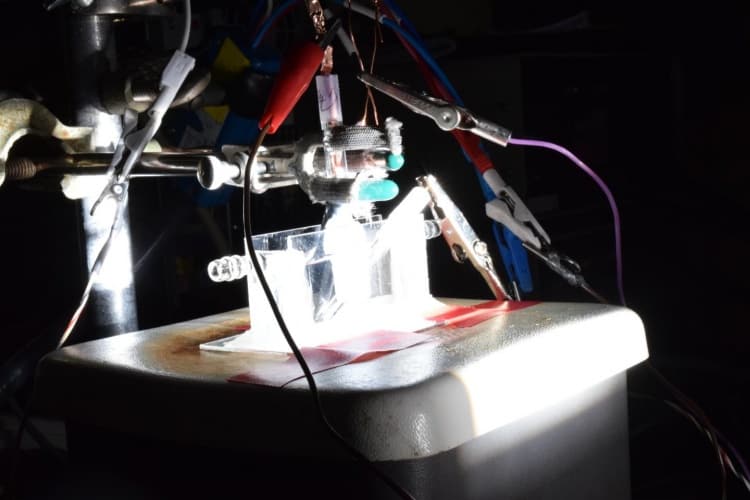
According to Prof Song Jin, who led the research team, the new catalyst is almost as efficient as platinum and appears to show the highest catalytic performance among the non-noble metal catalysts reported so far.
The advance emerges from a long line of research in Jin’s lab that has focused on the use of iron pyrite and other inexpensive, abundant materials for energy transformation. Jin and his students discovered the new catalyst by replacing iron to make cobalt pyrite, and then added phosphorus.
What’s more, although electricity is the usual energy source for splitting water into hydrogen and oxygen, Jin said that the new catalyst can also work with energy from sunlight. “We have demonstrated a proof-of-concept device for using this cobalt catalyst and solar energy to drive hydrogen generation,” he claimed.




Poll: Should the UK’s railways be renationalised?
I think that a network inclusive of the vehicles on it would make sense. However it remains to be seen if there is any plan for it to be for the...