Method utilises sunlight to create carbon-free energy
Chemists at the University of Rochester, New York, claim to have increased the output and lowered the cost of current light-driven hydrogen-production systems.
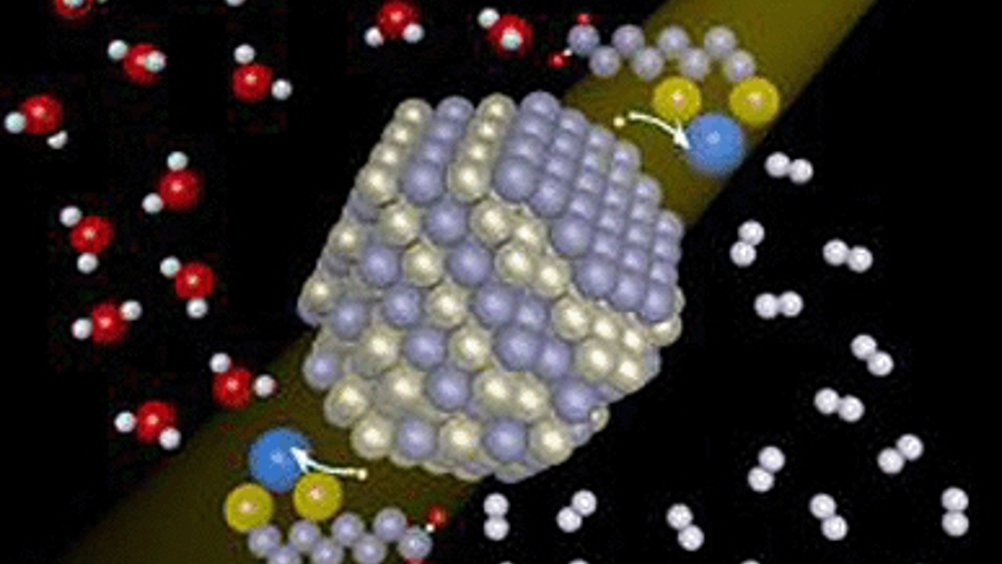
The work, funded by the US Department of Energy, was led by chemistry professors Richard Eisenberg, Todd Krauss, and Patrick Holland, and included graduate students Zhiji Han and Fen Qiu. Their paper will be published later this month in the journal Science.
In a statement, the chemists said their work efficiently uses sunlight to provide carbon-free energy for vehicles or anything requiring electricity in order to operate.
One disadvantage of current methods of hydrogen production has been the lack of durability in the light-absorbing material, but the Rochester scientists were able to overcome that problem by incorporating nanocrystals.
‘Organic molecules are typically used to capture light in photocatalytic systems,’ said Krauss. ‘The problem is they only last hours, or, if you’re lucky, a day. These nanocrystals performed without any sign of deterioration for at least two weeks.’
Eisenberg, the Tracy H Harris professor of chemistry, has spent two decades working on solar energy systems. During that time, his systems have typically generated 10,000 instances (turnovers) of hydrogen atoms being formed without having to replace any components. With the nanocrystals, Eisenberg and his colleagues witnessed turnovers in excess of 600,000.
Register now to continue reading
Thanks for visiting The Engineer. You’ve now reached your monthly limit of news stories. Register for free to unlock unlimited access to all of our news coverage, as well as premium content including opinion, in-depth features and special reports.
Benefits of registering
-
In-depth insights and coverage of key emerging trends
-
Unrestricted access to special reports throughout the year
-
Daily technology news delivered straight to your inbox










UK Enters ‘Golden Age of Nuclear’
The delay (nearly 8 years) in getting approval for the Rolls-Royce SMR is most worrying. Signifies a torpid and expensive system that is quite onerous...